Abstract
Nerve allografts provide a temporary scaffold for host nerve regeneration and allow for the repair of significant segmental nerve injuries. From rodent, large animal, and nonhuman primate studies, as well as clinical experience, nerve allografts, with the use of immunosuppression, have the capacity to provide equal regeneration and function to that of an autograft. In contrast to solid organ transplantation and composite tissue transfers, nerve allograft transplantation requires only temporary immunosuppression. Furthermore, nerve allograft rejection is difficult to assess, as the nerves are surgically buried and are without an immediate functional endpoint to monitor. In this article, we review what we know about peripheral nerve allograft transplantation from three decades of experience and apply our current understanding of nerve regeneration to the emerging field of composite tissue transplantation.
Keywords: Nerve allotransplantation, Composite tissue transplantation, Immunosuppression
Composite tissue allografting is an exciting field that can drastically change the lives of many individuals plagued with severe injuries. This emerging field takes into account the transplantation and immunology of multiple tissues. Peripheral nerve regeneration is critical for the function and sensation of these transplanted parts whether involving the face or an extremity. In this article, we review what we know about peripheral nerve allotransplantation from three decades of experience and apply our current understanding of nerve regeneration to the field of composite tissue transplantation.
Nerve Allografting
The repair of large, segmental, or complex nerve injuries requires equally long autogenous nerve grafts in which the supply is commonly insufficient for repair. When nerve continuity cannot be restored by a tension-free coaptation and donor autografts are insufficient, cadaveric nerve allografts provide a readily accessible alternative. Transplanted nerve allografts act as a temporary scaffold providing the substrate to permit host axonal regeneration. They also avoid graft site morbidity associated with nerve autografting such as sensory loss, scarring, and neuroma formation. The results of studies on rodent, large animal, and nonhuman primates, as well as clinical experience has demonstrated in the presence of immunosuppression nerve allografts provide equal regeneration and function to that of an autograft. However, due to the risks of temporary systemic immunosuppression (approximately 2 years) and the recent successful use of other autograft alternatives, such as nerve transfers or nerve transfers used in combination with autografting, the indication for nerve allotransplantation is relatively rare. Nerve allotransplantation should be reserved for unique patients with irreparable peripheral nerve injuries which left untreated would lead to an essentially nonfunctional limb.
In contrast to the allotransplantation of solid organs, nerve allografts require only temporary systemic immunosuppression [38]. Once axon regeneration has crossed the transplanted nerve allograft, systemic immunosuppression can be withdrawn [23, 38]. In organ transplantation, measurements of organ function, such as serum creatinine and liver transaminases, provide clinicians with an objective means to detect rejection. In contrast, rejection of nerve allografts is difficult to assess as the nerves are surgically buried and an immediate functional endpoint does not exist to alert one to rejection. In nerve allotransplantation, once rejection is detected, usually by overlying inflammation or erythema, it is usually too late for the rescue of the graft with modifications of the immunosuppression [1]. Thus, having clinical suspicion and ensuring patient compliance to the immunosuppressive regime is critical.
Nerve Allograft Immunosuppression with FK-506
Extensive and significant research has examined the immunology of nerve allograft transplantation [1, 2, 22, 24, 30, 43]. Previous studies have focused on minimizing the negative side effects of immunosuppression while maximizing nerve regeneration. Nerve allograft transplantation induces both a humoral and cell-mediated immune response [16, 26, 34, 50, 51]. In nerve allografts, Schwann cells (SCs) represent the main immunogenic target for acute and chronic rejection. SCs elicit host immune response through expression of both major histocompatibility complex (MHC) I and II [30, 34, 41, 52, 58].
Our laboratory began investigating the effects of immunosuppression on nerve regeneration and nerve allograft survival in the late 1970s and specifically looked at the calcineurin phosphatase inhibitor, FK-506, in the late 1990s. FK-506, also known as tacrolimus, ultimately inhibits the activation of T-cell proliferation [55]. FK-506 had a significant effect in solid organ transplant immunosuppression when it became approved for clinical use in the early 1990s [18, 42]. Gold et al. was first to describe augmented neuroregeneration provided by FK-506 in peripheral nerves [19]. FK-506 has since been shown to be effective in the enhancement of peripheral nerve regeneration in multiple models including transection [28], crush [31, 54], chronic axotomy [48], isograft [11], and allograft [14, 38] models. Beyond improved nerve regeneration, FK-506 has been shown to accelerate functional recovery in small and large animal models [27, 28]. The immunosuppressive mechanism of FK-506 is mediated through calcineurin-inhibition [8, 32], yet the exact mechanism of its neuroregenerative properties remains unclear. Evidence for calcineurin-independent pathway of action suggests that other mediators, such as FKBP-52, GAP43, heat shock proteins, and cytoskeletal dynamics may be involved [20, 29, 49].
Delays in administration of FK-506 should be avoided [44, 45]. Our clinical and research experience has demonstrated that FK-506 should be administered preoperatively for a greater effect on nerve regeneration and functional recovery. In 2006, we demonstrated that preloading FK-506 3 days before surgery enhanced the neuroregenerative effects of the drug [44]. We further demonstrated that if immunosuppression drops below therapeutic levels and rejection is suspected, the allograft can be rescued by providing immunosuppressive doses of FK-506 but only within 7 days of the start of rejection [14]. These observations have been translated to the senior author’s current clinical practice.
In the course of our investigations, we also discovered that low dose FK-506 in combination with other immunosuppressive regimes (anti-CD-40 ligand/co-stimulatory blockade and cold preservation in University of Wisconsin solution) continued to enhance peripheral nerve regeneration [5, 21]. In contrast, immunosuppressive doses of FK-506 with therapeutic doses of anti-CD-40 ligand/co-stimulatory blockade abrogates the beneficial effects of FK-506 on nerve regeneration [5]. Although not specifically studied, we suspect the decreased regenerative enhancement by FK-506 results from interference of each others mechanism of action (co-stimulatory blockade versus calcineurin inhibition), making both drugs less effective. For example, alloantigen needs to be recognized by host T cells to allow costimulation to be effective. Further studies are needed to elucidate the specifics behind this phenomenon.
Clinical Experience
Currently, the senior author has reserved nerve allografting for only devastating injuries which otherwise would not be reconstructable and would lead to total functional limb loss. The introduction of nerve transfers in the last several years has allowed for the treatment of more proximal and extensive nerve injuries, avoiding the need for nerve allotransplantation in some instances [9, 10, 35, 36, 39, 40]. Interestingly, decellularized cadaver nerve allografts are also now available commercially for use in nerve reconstructions. However, their use is limited to the repair of nerve gaps less than 3 cm in length and for small diameter nerves [57]. Experimental and clinical evidence for the use in longer gap lengths has not yet been demonstrated. Thus, we would only recommend the use of decellularized nerve allografts for short defects in small diameter nerves. Furthermore, while several commercially available synthetic absorbable and nonabsorbable nerve conduits exist, these too are limited by gap length and nerve diameter [6, 7, 37, 47]. Thus, in the event of truly devastating and extensive nerve injuries, the use of nerve allograft transplantation is the only viable and efficacious option.
Our current clinical regimen for nerve allotransplantation consists of using allografts taken from ABO blood-type-matched individuals as well as obvious available donor autografts. Recently, living related donors have been used in addition to cadaveric specimens. In one patient autograft, donor-related and donor-unrelated (noncadaveric) allografts were all used in the repair. We recommend using donor-related nerve allografts whenever possible because it shortens the wait time between the determination of the need for surgery and the actual operation. In our experience, a waiting period of 2–3 months to obtain cadaver nerves exists due to the current national system of tissue procurements.
In addition to the blood type compatibility, multiple small diameter nerves are used in contrast to larger diameter nerves to avoid necrosis of the central aspect of the nerve from lack of adequate revascularization. The allografts then undergo cold preservation in University of Wisconsin solution with antibiotics at 4°C for 7 days prior to implantation to decrease immunogenicity [13, 46]. Preoperatively, FK-506 is given to the patient 3 days prior to surgery for induction and to maximize the regenerative effects of the drug. Our current immunosuppressive drugs include the use of FK-506 and azathioprine. Our patient protocol is illustrated in Table 1.
Table 1.
Nerve allotransplantation patient protocol.
| Preoperative preparation | Intraoperative details | Postoperative care |
|---|---|---|
| Assess patient suitability | Resect patient’s injured nerve to healthy proximal and distal stumps | Induction immunotherapy (dose 2) |
| Medical comorbidities | Basiliximab 20 mg intravenously (postoperative day 4) | |
| Infection/malignancy | ||
| Psychosocial fitness | ||
| Laboratory tests | Use patient’s autografts in addition to cadaveric allografts, donor-related or donor-unrelated allografts | Standard immunotherapy |
| ABO blood type | continue FK-506 (goal level 5–8 ng/ml) | |
| CBC, CMP | Azathioprine 1–1.5 mg/kg/day | |
| HIV/hepatitis | ||
| Nerve allografts | Insert extra allograft subcutaneously for rejection monitoring | Antibiotics |
| Consider related donor | Sulfamethoxazole-trimethoprim | |
| Harvest small diameter grafts | Three times a week | |
| Cold preservation (4°C, University of Wisconsin solution, 7 days | ||
| Immunotherapy | Stop immunotherapy | |
| FK-506 (tacrolimus) 1–2 mg by mouth B. I.D. (start 3 days preoperatively) | 6 months after Tinels crosses distal repair site | |
| Induction immunotherapy (dose 1)—Basiliximab 20 mg intravenously (immediately preoperatively) |
Modified from I. K. Fox and S. E. Mackinnon. Seminars in Plastics Surgery/Vol 21, Number 4 2007 [13]
The senior author has published her cases of human nerve allotransplantation (for review see [38]). Since 1988, 11 patients have been treated with a combination of autografts and donor allografts. In the two most recent patients, living related donors have been used in addition to cadaveric specimens. In one patient, autograft, donor-related and donor-unrelated (noncadaveric) allografts were all used in the repair. Ten of the 11 patients have had a good recovery, while a single patient developed rejection following subtherapeutic dosing with FK506.
Schwann Cell Migration
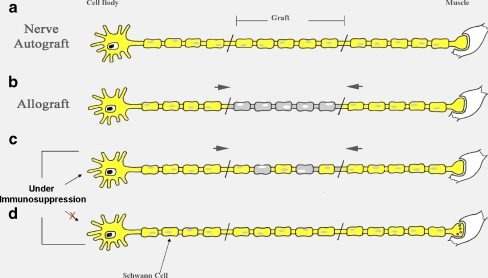
Host SC migration is essential for nerve allograft survival and axonal regeneration through the graft. In order to minimize immunosuppressive therapy, both host and donor SC migration and viability are critical. Studies exploring SC behavior and migration have demonstrated that host SCs migrate into the nerve allograft from both proximal and distal host nerve ends (Fig. 1) [15, 17, 27, 53]. Observations of in vivo imaging show that there appears to be more migration from the distal host nerve. Host SCs repopulate an accellularized graft proximal and distally very early (within 10 days) and axonal regeneration lags behind SC migration by 10–15 days [27].
Figure 1.
Schwann cell migration. In the nerve autograft (a), Schwann cells (SCs) in the graft support the survival. b In the nerve allograft, host Schwann cells (SCs) migrate from both proximal and distal host nerve into the donor graft. c Under adequate immunosuppression, the host SCs coexist with donor SCs within the allograft and support axonal regeneration through the graft. d Once immunosuppression is stopped, the host SCs migrate across the entire graft and replace the donor SCs.
Recent studies in our laboratory have further explored SC migration into a nerve allograft. We determined that under adequate immunosuppression there is a delay in host SC migration across the entire graft. During this delay, the donor SCs appear to assist axonal regeneration across the graft. [56]. Over time, host SCs migrate into the graft and both host and donor SCs coexist. This movement of host SCs across the graft may be related to episodes of subclinical rejection because once immunosuppression is stopped, we immediately see host SC migration into graft [56]. These findings shed insight into the support and survival of nerve allografts by SCs and become inherently important when considering the survival of nerves in composite tissue transplantation.
Nerves in Composite Tissue Allotransplantation
Composite tissue allografts represent a unique challenge for long-term nerve allograft viability. Ultimately, the long-term functional outcome in patients undergoing either hand or face transplantation requires appropriate and sufficient nerve regeneration. Neuromuscular recovery as well as sensory function have been described in both small animal models [3, 4] and primate models [12, 25] undergoing composite tissue transfers. More recently, Cottrell et al. observed fewer axons with a higher percentage of myelination in transplanted nerves in rats undergoing hind limb transplantation. In nerve allotransplantation, allograft survival largely depends upon both proximal and distal SC migration during axonal regeneration with end-organ function dependent upon autologous axonal regeneration. However, in composite tissue allotransplantion, critical distal host SCs do not exist. Thus, is the observed restoration of function and robust myelination found in composite grafts due solely to migrating host SCs or are donor SCs responsible for myelination and return of function? Or is host uninjured neuromuscular function proximal to the composite tissue allograft responsible for return of function? Will host SCs migrate to encompass the entire nerve in the environment of long-term immunosuppression?
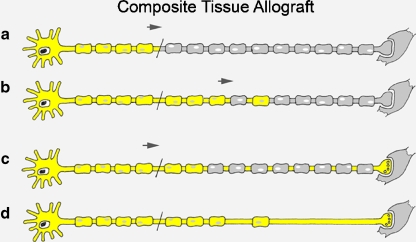
The answers to the behavior of host and donor SC migration in composite tissue allotransplantation have not yet been elucidated. Our current research is directed at exploring specifically nerve regeneration and the influence of SCs in limb allotransplantation. However, from our understanding of nerve allotransplantation, we believe that the nerves in composite tissue transfers need perfect SC support for regeneration. This may require no lapses in immunosuppression to prevent loss of the donor SCs. Without the migration of distal host SCs, donor SCs are critical for the axonal regeneration and end-organ function. If the nerve is demyelinated distally with loss of distal SCs, then neurapraxia and potentially complete and permanent conduction block may occur resulting in permanent functional loss [33] (Fig. 2). Thus, effective immunosuppression and the avoidance of rejection are critical for the survival of donor SCs and the ultimate survival and function of the composite tissue transplant.
Figure 2.
Schwann cell migration in composite tissue allotransplantation. a In the composite tissue transplanted nerve, there are no distal host Schwann cells (SC). b SC migration occurs only from proximal end. c With adequate immunosuppression, both host and donor SCs will support axonal regeneration and allow for end organ reinnervation. d If episodes of rejection occur and donor SCs are lost, neurapraxia may ensue and potentially a complete and permanent conduction block may occur resulting in no return of function.
Conclusion
Adequate neural regeneration and end-organ function is critical for composite tissue allotransplantation function. Unfortunately, there are many unanswered questions still remaining. Understanding the history and experience with nerve allotransplantation, as well as, future work focusing specifically on the impact of SC migration and neural regeneration is necessary to improve outcomes.
Footnotes
No benefit of any kind will be received either directly or indirectly by the authors.
Grant Funding: National Institute of Health 5RO1NS033406-14.
References
- 1.Argall KG, Armati PJ, Pollard JD, Watson E, Bonner J. Interactions between CD4+ T-cells and rat Schwann cells in vitro. 1. Antigen presentation by Lewis rat Schwann cells to P2-specific CD4+ T-cell lines. J Neuroimmunol 1992;40:1–18. doi:10.1016/0165-5728(92)90208-3. [DOI] [PubMed]
- 2.Atchabahian A, Mackinnon SE, Hunter DA. Cold preservation of nerve grafts decreases expression of ICAM-1 and class II MHC antigens. J Reconstr Microsurg 1999;15:307–11. [DOI] [PubMed]
- 3.Black KS, Hewitt CW, Fraser LA, Howard EB, Martin DC, Achauer BM, et al. Composite tissue (limb) allografts in rats. II. Indefinite survival using low-dose cyclosporine. Transplantation 1985;39:365–8. doi:10.1097/00007890-198504000-00005. [DOI] [PubMed]
- 4.Black KS, Hewitt CW, Grisham GR, Caiozzo VJ, Howard EB, Achauer BM. Two new composite tissue allograft models in rats to study neuromuscular functional return. Transplant Proc 1987;19:1118–9. [PubMed]
- 5.Brenner MJ, Mackinnon SE, Rickman SR, Jaramillo A, Tung TH, Hunter DA, et al. FK506 and anti-CD40 ligand in peripheral nerve allotransplantation. Restor Neurol Neurosci 2005;23:237–49. [PubMed]
- 6.Chiu DT, Janecka I, Krizek TJ, Wolff M, Lovelace RE. Autogenous vein graft as a conduit for nerve regeneration. Surgery 1982;91:226–33. [PubMed]
- 7.Chiu DT, Lovelace RE, Yu LT, Wolff M, Stengel S, Middleton L, et al. Comparative electrophysiologic evaluation of nerve grafts and autogenous vein grafts as nerve conduits: an experimental study. J Reconstr Microsurg 1988;4:303–9, 311–302. [DOI] [PubMed]
- 8.Clipstone NA, Crabtree GR. Calcineurin is a key signaling enzyme in T lymphocyte activation and the target of the immunosuppressive drugs cyclosporin A and FK506. Ann N Y Acad Sci 1993;696:20–30. [DOI] [PubMed]
- 9.Colbert SH, Mackinnon S. Posterior approach for double nerve transfer for restoration of shoulder function in upper brachial plexus palsy. Hand 2006;1:71–7. doi:10.1007/s11552-006-9004-4. [DOI] [PMC free article] [PubMed]
- 10.Colbert SH, Mackinnon SE. Nerve transfers for brachial plexus reconstruction. Hand Clin 2008;24:341–61. doi:10.1016/j.hcl.2008.07.001. [DOI] [PubMed]
- 11.Doolabh VB, Mackinnon SE. FK506 accelerates functional recovery following nerve grafting in a rat model. Plast Reconstr Surg 1999;103:1928–36. doi:10.1097/00006534-199906000-00018. [DOI] [PubMed]
- 12.Egerszegi EP, Samulack DD, Daniel RK. Experimental models in primates for reconstructive surgery utilizing tissue transplants. Ann Plast Surg 1984;13:423–30. doi:10.1097/00000637-198411000-00010. [DOI] [PubMed]
- 13.Evans PJ, MacKinnon SE, Midha R, Wade JA, Hunter DA, Nakao Y, et al. Regeneration across cold preserved peripheral nerve allografts. Microsurgery 1999;19:115–27. doi:10.1002/(SICI)1098-2752(1999)19:3<115::AID-MICR1>3.0.CO;2-9. [DOI] [PubMed]
- 14.Feng FY, Ogden MA, Myckatyn TM, Grand AG, Jensen JN, Hunter DA, et al. FK506 rescues peripheral nerve allografts in acute rejection. J Neurotrauma 2001;18:217–29. doi:10.1089/08977150150502631. [DOI] [PubMed]
- 15.Fornaro M, Tos P, Geuna S, Giacobini-Robecchi MG, Battiston B. Confocal imaging of Schwann-cell migration along muscle-vein combined grafts used to bridge nerve defects in the rat. Microsurgery 2001;21:153–5. doi:10.1002/micr.1029. [DOI] [PubMed]
- 16.Fox IK, Mackinnon SE. Experience with nerve allograft transplantation. Semin Plast Surg 2007;21:242–9. doi:10.1055/s-2007-991194. [DOI] [PMC free article] [PubMed]
- 17.Fukaya K, Hasegawa M, Mashitani T, Kadoya T, Horie H, Hayashi Y, et al. Oxidized galectin-1 stimulates the migration of Schwann cells from both proximal and distal stumps of transected nerves and promotes axonal regeneration after peripheral nerve injury. J Neuropathol Exp Neurol 2003;62:162–72. [DOI] [PubMed]
- 18.Fung JJ, Starzl TE. FK 506 in solid organ transplantation. Transplant Proc 1994;26:3017–20. [PubMed]
- 19.Gold BG, Katoh K, Storm-Dickerson T. The immunosuppressant FK506 increases the rate of axonal regeneration in rat sciatic nerve. J Neurosci 1995;15:7509–16. [DOI] [PMC free article] [PubMed]
- 20.Gold BG, Zeleny-Pooley M, Wang MS, Chaturvedi P, Armistead DM. A nonimmunosuppressant FKBP-12 ligand increases nerve regeneration. Exp Neurol 1997;147:269–78. doi:10.1006/exnr.1997.6630. [DOI] [PubMed]
- 21.Grand AG, Myckatyn TM, Mackinnon SE, Hunter DA. Axonal regeneration after cold preservation of nerve allografts and immunosuppression with tacrolimus in mice. J Neurosurg 2002;96:924–32. [DOI] [PubMed]
- 22.Gulati AK. Immune response and neurotrophic factor interactions in peripheral nerve transplants. Acta Haematol 1998;99:171–4. doi:10.1159/000040832. [DOI] [PubMed]
- 23.Hayashi A, Koob JW, Liu DZ, Tong AY, Hunter DA, Parsadanian A, et al. A double-transgenic mouse used to track migrating Schwann cells and regenerating axons following engraftment of injured nerves. Exp Neurol 2007;207:128–38. doi:10.1016/j.expneurol.2007.06.004. [DOI] [PMC free article] [PubMed]
- 24.Hettiaratchy S, Melendy E, Randolph MA, Coburn RC, Neville DM Jr, Sachs DH, et al. Tolerance to composite tissue allografts across a major histocompatibility barrier in miniature swine. Transplantation 2004;77:514–21. doi:10.1097/01.TP.0000113806.52063.42. [DOI] [PubMed]
- 25.Hovius SE, Stevens HP, van Nierop PW, Rating W, van Strik R, van der Meulen JC. Allogeneic transplantation of the radial side of the hand in the rhesus monkey: I. Technical aspects. Plast Reconstr Surg 1992;89:700–9. doi:10.1097/00006534-199204000-00020. [DOI] [PubMed]
- 26.Ishida O, Ochi M, Ikuta Y, Akiyama M. Peripheral nerve allograft: cellular and humoral immune responses of mice. J Surg Res 1990;49:233–8. doi:10.1016/0022-4804(90)90125-L. [DOI] [PubMed]
- 27.Jensen JN, Brenner MJ, Tung TH, Hunter DA, Mackinnon SE. Effect of FK506 on peripheral nerve regeneration through long grafts in inbred swine. Ann Plast Surg 2005;54:420–7. doi:10.1097/01.sap.0000151461.60911.c0. [DOI] [PubMed]
- 28.Jost SC, Doolabh VB, Mackinnon SE, Lee M, Hunter D. Acceleration of peripheral nerve regeneration following FK506 administration. Restor Neurol Neurosci 2000;17:39–44. [PubMed]
- 29.Klettner A, Baumgrass R, Zhang Y, Fischer G, Burger E, Herdegen T, et al. The neuroprotective actions of FK506 binding protein ligands: neuronal survival is triggered by de novo RNA synthesis, but is independent of inhibition of JNK and calcineurin. Brain Res 2001;97:21–31. doi:10.1016/S0169-328X(01)00286-8. [DOI] [PubMed]
- 30.Lassner F, Schaller E, Steinhoff G, Wonigeit K, Walter GF, Berger A. Cellular mechanisms of rejection and regeneration in peripheral nerve allografts. Transplantation 1989;48:386–92. doi:10.1097/00007890-198909000-00006. [DOI] [PubMed]
- 31.Lee M, Doolabh VB, Mackinnon SE, Jost S. FK506 promotes functional recovery in crushed rat sciatic nerve. Muscle Nerve 2000;23:633–40. doi:10.1002/(SICI)1097-4598(200004)23:4<633::AID-MUS24>3.0.CO;2-Q. [DOI] [PubMed]
- 32.Liu J, Farmer JD Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 1991;66:807–15. doi:10.1016/0092-8674(91)90124-H. [DOI] [PubMed]
- 33.Liu DZ, Tung TH, Mackinnon SE. Nerve transfer for elbow flexion in radiation-induced brachial plexopathy: A case report. Hand. 2009 (in press). [DOI] [PMC free article] [PubMed]
- 34.Mackinnon S, Hudson A, Falk R, Bilbao J, Kline D, Hunter D. Nerve allograft response: a quantitative immunological study. Neurosurgery 1982;10:61–9. doi:10.1097/00006123-198201000-00011. [DOI] [PubMed]
- 35.Mackinnon SE. Preliminary results of double nerve transfer to restore elbow flexion in upper type brachial plexus palsies. Plast Reconstr Surg 2006;118:1273. author reply 1274. [DOI] [PubMed]
- 36.Mackinnon SE, Colbert SH. Nerve transfers in the hand and upper extremity surgery. Tech Hand Up Extrem Surg 2008;12:20–33. [DOI] [PubMed]
- 37.Mackinnon SE, Dellon AL. A study of nerve regeneration across synthetic (Maxon) and biologic (collagen) nerve conduits for nerve gaps up to 5 cm in the primate. J Reconstr Microsurg 1990;6:117–21. [DOI] [PubMed]
- 38.Mackinnon SE, Doolabh VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg 2001;107:1419–29. doi:10.1097/00006534-200105000-00016. [DOI] [PubMed]
- 39.Mackinnon SE, Roque B, Tung TH. Median to radial nerve transfer for treatment of radial nerve palsy. Case report. J Neurosurg 2007;107:666–71. doi:10.3171/JNS-07/09/0666. [DOI] [PubMed]
- 40.Novak CB, Mackinnon SE. Treatment of a proximal accessory nerve injury with nerve transfer. Laryngoscope 2004;114:1482–4. doi:10.1097/00005537-200408000-00030. [DOI] [PubMed]
- 41.Pollard JD, Gye RS, McLeod JG. An assessment of immunosuppressive agents in experimental peripheral nerve transplantation. Surg Gynecol Obstet 1971;132:839–45. [PubMed]
- 42.Shapiro R. Tacrolimus in solid organ transplantation: an update. Transplant Proc 1999;31:2203–5. doi:10.1016/S0041-1345(99)00306-1. [DOI] [PubMed]
- 43.Siemionow M, Sonmez E. Nerve allograft transplantation: a review. J Reconstr Microsurg 2007;23:511–20. doi:10.1055/s-2007-1022694. [DOI] [PubMed]
- 44.Snyder AK, Fox IK, Nichols CM, Rickman SR, Hunter DA, Tung TH, et al. Neuroregenerative effects of preinjury FK-506 administration. Plast Reconstr Surg 2006;118:360–7. doi:10.1097/01.prs.0000227628.43867.5b. [DOI] [PubMed]
- 45.Sobol JB, Lowe IJ, Yang RK, Sen SK, Hunter DA, Mackinnon SE. Effects of delaying FK506 administration on neuroregeneration in a rodent model. J Reconstr Microsurg 2003;19:113–8. doi:10.1055/s-2003-37817. [DOI] [PubMed]
- 46.Strasberg SR, Hertl MC, Mackinnon SE, Lee CK, Watanabe O, Tarasidis G, et al. Peripheral nerve allograft preservation improves regeneration and decreases systemic cyclosporin A requirements. Exp Neurol 1996;139:306–16. doi:10.1006/exnr.1996.0104. [DOI] [PubMed]
- 47.Strauch B, Ferder M, Lovelle-Allen S, Moore K, Kim DJ, Llena J. Determining the maximal length of a vein conduit used as an interposition graft for nerve regeneration. J Reconstr Microsurg 1996;12:521–7. [DOI] [PubMed]
- 48.Sulaiman OA, Voda J, Gold BG, Gordon T. FK506 increases peripheral nerve regeneration after chronic axotomy but not after chronic schwann cell denervation. Exp Neurol 2002;175:127–37. doi:10.1006/exnr.2002.7878. [DOI] [PubMed]
- 49.Tanaka K, Fujita N, Higashi Y, Ogawa N. Neuroprotective and antioxidant properties of FKBP-binding immunophilin ligands are independent on the FKBP12 pathway in human cells. Neurosci Lett 2002;330:147–50. doi:10.1016/S0304-3940(02)00755-3. [DOI] [PubMed]
- 50.Trumble T, Gunlikson R, Parvin D. A comparison of immune response to nerve and skin allografts. J Reconstr Microsurg 1993;9:367–72. [DOI] [PubMed]
- 51.Trumble TE, Gunlikson R, Parvin D. Systemic immune response to peripheral nerve transplants across major histocompatibility class-I and class-II barriers. J Orthop Res 1994;12:844–52. doi:10.1002/jor.1100120612. [DOI] [PubMed]
- 52.Trumble TE, Shon FG. The physiology of nerve transplantation. Hand Clin 2000;16:105–22. [PubMed]
- 53.Tseng CY, Hu G, Ambron RT, Chiu DT. Histologic analysis of Schwann cell migration and peripheral nerve regeneration in the autogenous venous nerve conduit (AVNC). J Reconstr Microsurg 2003;19:331–40. doi:10.1055/s-2003-42502. [DOI] [PubMed]
- 54.Udina E, Ceballos D, Gold BG, Navarro X. FK506 enhances reinnervation by regeneration and by collateral sprouting of peripheral nerve fibers. Exp Neurol 2003;183:220–31. doi:10.1016/S0014-4886(03)00173-0. [DOI] [PubMed]
- 55.Wang MS, Zeleny-Pooley M, Gold BG. Comparative dose-dependence study of FK506 and cyclosporin A on the rate of axonal regeneration in the rat sciatic nerve. J Pharmacol Exp Ther 1997;282:1084–93. [PubMed]
- 56.Whitlock EL, Myckatyn TM, Tony AY, Yee AX, Yan Y, Moore AM, et al. Schwann cell migration into peripheral nerve allografts: a longitudinal assessment. Exp Neurol. 2009 (in press). [DOI] [PMC free article] [PubMed]
- 57.Whitlock EL, Tuffaha SH, Luciano JP, Yan Y, Hunter DH, Magill CK, et al. Processed Allografts and Type I Collagen Conduits for Repair of Peripheral Nerve Gaps. Muscle Nerve. 2009 (in press). [DOI] [PubMed]
- 58.Yu LT, Rostami A, Silvers WK, Larossa D, Hickey WF. Expression of major histocompatibility complex antigens on inflammatory peripheral nerve lesions. J Neuroimmunol 1990;30:121–8. doi:10.1016/0165-5728(90)90095-5. [DOI] [PubMed]