Abstract
Excessive adiposity is associated with increased oxidative stress and accelerated aging. Weight loss induced by negative energy balance reduces markers of oxidation in experimental animals and humans. The long-term effects of weight loss induced by calorie restriction or increased energy expenditure induced by exercise on measures of oxidative stress and damage have not been studied in humans. The objective of the present study was to compare the effects of 20% caloric restriction or 20% exercise alone over 1 year on oxidative damage to DNA and RNA, as assessed through white blood cell and urine analyses. Eighteen men and women aged 50 to 60 years with a body mass index (BMI) between 23.5 to 29.9 kg/m2 were assigned to one of two conditions — 20% CR (n = 9) or 20% EX (n = 9) — which was designed to produce an identical energy deficit through increased energy expenditure. Compared to baseline, both interventions significantly reduced oxidative damage to both DNA (48.5% and 49.6% reduction for the CR and EX groups, respectively) and RNA (35.7% and 52.1% reduction for the CR and EX groups, respectively) measured in white blood cells. However, urinary levels of DNA and RNA oxidation products did not differ from baseline values following either 12-month intervention program. Data from the present study provide evidence that negative energy balances induced through either CR or EX result in substantial and similar improvements in markers of DNA and RNA damage to white blood cells, potentially by reducing systemic oxidative stress.
Introduction
Oxidative damage to DNA, proteins, lipids, and other cellular components accumulates over time and has been hypothesized to be a major cause of aging and age-associated diseases.1,2 In support of this hypothesis, accelerated aging and cellular oxidative damage have been linked in rodents.3 Experimental evidence indicates that excessive adiposity is associated with increased oxidative stress.4 In contrast, weight loss in obese patients causes a significant reduction in markers of oxidation, such as urinary 8-iso-PGF2α and protein carbonylation.5,6
Negative energy balance can be achieved by reducing energy intake or increasing energy expenditure. In rodents, both caloric restriction (CR) and exercise training have been found to reduce oxidative damage to lipids, protein, and DNA in many tissues.7–9 Calorie restriction has consistently been shown to extend lifespan and reduce age-related diseases in numerous species.10 Exercise training, however, has only been found to increase average lifespan and does not affect maximal lifespan.11 One explanation for these disparate effects is that exercise training may increase oxidative damage in some instances.12,13 Recent reports, however, indicate that exercise training does not increase oxidative damage in weight-matched rodents.14 Thus, the literature is currently mixed regarding the effect of exercise on oxidative damage. Moreover, the effects of long-term negative energy balance induced by either caloric restriction or exercise alone for reducing markers of oxidative stress in humans are not known.
We conducted a one-year randomized controlled trial in middle-aged lean and overweight men and women to evaluate the effect of body fat reduction induced by a 20% decrease in energy intake alone or 20% increase in energy expenditure alone on markers of DNA and RNA damage. We hypothesized that both calorie restriction (CR) and exercise (EX) would reduce DNA and RNA oxidative damage, but that these changes would be more pronounced when the energy deficit was achieved through CR than through EX.
Method
The current study represents an ancillary project of the main study entitled Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE, phase 1). This study was approved by the Human Studies Committee and the General Clinical Research Center Scientific Advisory Committee of Washington University School of Medicine. All subjects gave their informed consent before their participation.
Participants
An extensive screening process was employed in the CALERIE study to ensure participants were healthy and suitable for participation in this trial. Details of the screening process have been previously reported.15 Briefly, men and women aged 50 to 60 years with a body mass index (BMI) between 23.5 to 29.9 kg/m2 were recruited. Potential participants were excluded for the following reasons: (1) a history of diabetes or a fasting blood glucose value ≥126 mg/dl, (2) a history or clinical evidence of coronary artery disease, stroke, or lung disease, (3) a resting blood pressure (BP) ≥170 mmHg systolic or ≥100 mmHg diastolic, or (4) a recent history or evidence of malignancy. Furthermore, all candidates had to be nonsmokers and sedentary (defined as exercising <40 min per week during the 6 months before baseline testing). Women had to be postmenopausal.
Study design
For the larger study, eligible participants were randomized, with stratification for sex, to one of three groups in a 2:2:1 sequence: caloric restriction (CR) group (n = 19), exercise (EX) group (n = 19), or healthy lifestyle (HL) control group (n = 10) for 1 year. For the purpose of the current study, data were analyzed only for participants assigned to the CR and EX groups. The group samples were randomized and batch analyses were performed under the same conditions (see DNA and RNA oxidation section). Because one subject dropped out of each group before completing the study and because biological specimens were not available for all subjects, sample sizes for this ancillary study are smaller than those reported previously for the Washington University CALERIE study (sample sizes for each outcome are provided in the table and figures).
CR intervention
The goal of the CR intervention was to create a 20% energy deficit through a reduction in energy intake (without changing physical activity levels) for the duration of the 1 year intervention. Participants were instructed to decrease energy intake by 16% during the first 3 months and by 20% during the remaining 9 months. Diet prescriptions were based on participants' baseline energy intake, which was assumed to be equal to total daily energy expenditure as determined by the doubly labeled water (DLW) method.15 To enhance compliance to the intervention, participants met with registered dietitians on a weekly basis to discuss and review strategies for reducing energy intake, as well as attended weekly group meetings led by a dietitian and a behavioral psychologist. Further details about the CR intervention, including compliance data, have been reported previously.15
Exercise intervention
The goal of the EX intervention was to induce an energy deficit identical to the CR intervention by increasing daily energy expenditure through physical activity without changing caloric intake. Participants were instructed to increase energy expenditure by 16% of baseline total daily energy expenditure for the first 3 months and by 20% for the subsequent 9 months. They were informed that exercise sessions could be completed in one or several daily bouts, and exercise trainers worked closely with participants to monitor their energy expenditure goals. The participants exercised while using heart rate monitors (Polar S610, Polar Electro Oy, Kempele, Finland), which provided estimates of energy expenditure during exercise. To ensure participants' energy intake remained stable, the study dietitians periodically monitored energy intake using 7-day food diaries and provided consultation as needed. Additional details about the EX intervention, including compliance data, have been reported previously.15–17
Weight measurements
Body weight was measured twice in the morning following a 12 h fast. At baseline, weight was calculated from the mean of four weights measured over a 4-week baseline period. Twelve-month body weights represent the mean of three weekly weights obtained at the beginning, middle, and end of the 2-week assessment periods.
DNA and RNA oxidation
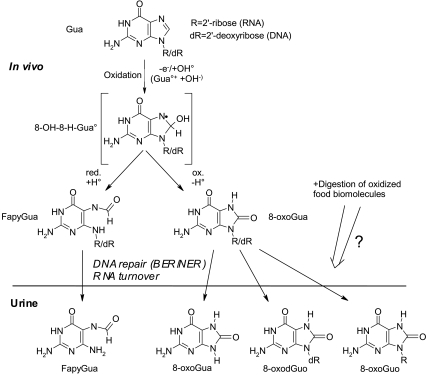
Urine analysis. Urine samples were collected for 12 h overnight from the participants using standardized procedures, aliquoted and frozen under argon at −80°C until analysis.18 The urinary RNA and DNA oxidative damage products 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyGua), 8-oxo-7,8-dihydroguanine (8-oxoGua), 8-oxo-7,8-dihydroguanosine (8-oxoGuo), and 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo) (chemical structures are shown in Fig. 1) were simultaneously measured for participants on CR and exercise employing electrospray tandem mass spectrometry detection (MS/MS) in multiple reaction monitoring (MRM) mode on a Finnigan TSQ 7000 triple quadrupole mass spectrometer (Thermo Electron Corporation, San Jose, CA). This method does not require any sample preparation except for the addition of buffer and 13C- and 15N-labeled internal standards (isotope dilution) to the urine prior to sample injection into the HPLC-MS/MS system, as described in detail elsewhere.18 The urinary biomarkers were normalized to the concentration of creatinine, which was assessed using a creatinine kit (Cayman Chemical, Ann Arbor, MI). The manufacturer's instructions were followed and the formation of acid-sensitive chromogen after reduction of the sample with picrate was measured spectrophotometrically at 500 nm.
FIG. 1.
Guanine oxidation reaction scheme with structures of nucleosides and bases analyzed using HPLC-ECD and HPLC-MS/MS. The reaction mechanism of guanine (Gua) with oxidizing agents, such as one-electron oxidants (produces a Gua•+ cation that can react with a hydroxyl anion, OH−) and the hydroxyl radical (OH•), forms an unstable intermediate radical (8-OH-H-Gua•) upon reaction at the exposed C-8 position.19,20 The reduction reaction (+e− +H+ (= +H•)) generates an 8-OH-H-Gua• ring opening to form FapyGua, whereas upon oxidation (-H•) 8-oxoGua is formed.19,20 Base and nucleotide excision repair (BER/NER) systems constantly proof DNA for mutagenic oxidation products to be removed from the cells and excreted into the plasma and filtered by the kidney. How oxidation products in RNA are removed is presently unclear, but is believed to occur by yet largely undefined mechanisms turning over damaged RNA.21 8-oxoGua and FapyGua can be excreted as free bases or attached to the sugar moieties present in RNA and DNA (R = ribose, dR = 2′-deoxyribose). FapyGua forms several isomers when attached to a sugar moiety and was measured as free base only. Urinary nucleoside oxidation products may to a minor extent also be absorbed from digested food or gut microbial end products, or stem from dead cells.22–24
Blood analyses. A venous blood sample was taken after participants had fasted for at least 12 h at baseline and at 12 months. In the EX group, blood samples were obtained at least 48 h after the last exercise session. Blood was collected in 10 mL EDTA-Vacutainer tubes from Becton Dickinson (Franklin Lakes, NJ), and white blood cell (WBC) “buffy coats” were collected following centrifugation (800 g, 20 min, 4°C) using a large orifice pipette tip and placed into 1.5 mL Eppendorf tubes and immediately frozen at −80°C. RNA and DNA oxidative damage levels to WBC were measured in the CR (n = 9) and EX (n = 9) subjects. Buffy coats of WBC were thawed from −80°C and placed on ice. Working on slush ice (0°C) during all steps, cells were lysed in 4.5 mL of 3 M GTC buffer (0.2 wt.% N-L-Sarcosine, 20 mM tris [pH 7.5]) containing 10 mM of the freshly dissolved metal chelator deferoxamine meylate (DFOM) during homogenization using a Potter-Elvehjem homogenizer. All chemicals and supplies used to extract and analyze nucleic acids were as previously described.25 After transferring the homogenates to 15 mL Phase-Lock Gel (PLG) tubes, an equal amount of a phenolchloroform mixture (pH 6.7) was added, and proteins and lipids extracted into the phenol phase. After vortexing in intervals of 10 min keeping the tubes on ice, samples were centrifuged (2000 g, 30 min, 0°C), and the upper aqueous phase containing nucleic acids was transferred into a new PLG tube. The procedure was repeated once. After transferring into a new PLG tube, an equal amount of chloroform-isoamylalcohol (24:1) was added to remove any remaining phenol by hand mixing followed by centrifugation. The procedure was repeated once, and the upper aqueous phase was collected and nucleic acids precipitated by adding 1:1 isopropanol, mixing and incubating at −80°C overnight. Total nucleic acids were collected by centrifugation at 10,000 g, 0°C for 10 min. Nucleic acids were washed in 70% ethanol, spun down at 3000 g (10 min, 0°C), and air-dried at room temperature for 10 min. RNA/DNA hydrolysis was performed using Nuclease P1 and alkaline phosphatase, and 8-oxoGuo/guanosine (Guo) and 8-oxodGuo/2′-deoxyguanosine (dGuo) ratios were determined using HPLC-ECD with a Coulochem III electrochemical detector (ESA Inc., Chelmsford, MA), as described previously.25
Statistical analysis
Baseline characteristics of participants were compared between groups using Fisher's exact test for categorical data and independent t-tests for quantitative data. One-way ANOVA and paired t test were performed to assess within-group changes, and analysis of covariance (ANCOVA) was used for between group comparisons after adjustment for initial values with subsequent Kruskal-Wallis test for post-hoc comparisons. All statistical tests were two-tailed, and significance was accepted at p < 0.05. Data are presented as means ± standard error (SE) at each time point, and for the change between baseline and 12 months. All analyses were performed using Prism 4 from GraphPad software (San Diego, CA).
Results
Participants
Subject characteristics data represent all participants who were included in the WBC- or urine-based analyses (N = 34). Male/female representation in the CR (7 men, 10 women) and EX (5 men, 12 women) groups did not differ significantly (p = 0.72). The participants in the CR group were slightly younger than those in the EX group (54.6 ± 3.1 vs. 58.6 ± 2.7 years, p = 0.0004), although all participants were within the 50 to 60 year range required for the study. Average BMI was in the overweight range (CR, 26.8 ± 2.4 kg/m2; EX, 26.7 ± 1.8 kg/m2) and did not differ between groups (p = 0.89). Results from statistical analyses performed on each subgroup alone (i.e., participants included in the WBC-based analyses (n = 18) or those included in the urine-based analyses (n = 29) were not different from analyses conducted on the entire sample.
Body weight
Baseline body weight was similar in the CR (79.1 ± 9.8 kg) and exercise (75.6 ± 10.3 kg) groups and did not differ between groups (p = 0.32). Body weight decreased in both groups (CR, −10.2 ± 1.5%, p < 0.0001; EX, −8.0 ± 1.5%, p < 0.0001) and these decreases did not differ between groups (p = 0.31). These results are based on subjects who were included in either the WBC- or urine-based data analyses (n = 34); statistical results from analyses on either subgroup alone were not different from the results from all subjects combined (data not shown).
RNA and DNA oxidative damage
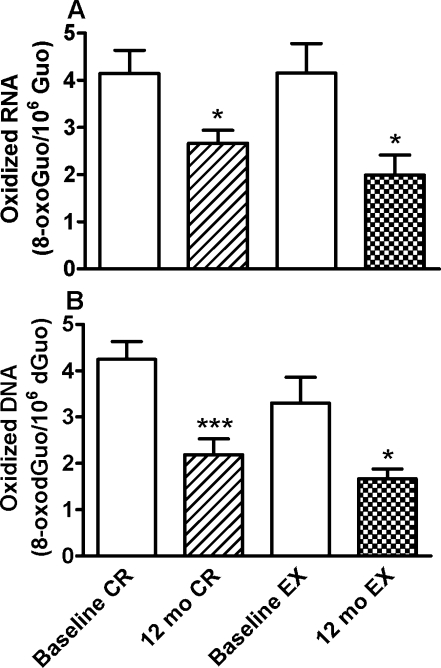
Analyses of white blood cells. The two groups did not differ in levels of RNA or DNA oxidative damage at baseline. As presented in Figure 2, both interventions significantly reduced oxidative damage to both DNA and RNA. Levels of WBC DNA oxidation (8-oxodGuo/ 106 dGuo) decreased by 48.5% from baseline (4.24 ± 0.39) to 1 year (2.19 ± 0.34) for the CR group, and by 49.6% from baseline (3.30 ± 0.56) to 1 year (1.66 ± 0.22) for the EX group. Similarly, levels of total WBC RNA oxidation expressed as 8-oxoGuo/106 Guo decreased by 35.7% from baseline (4.15 ± 0.49) to 1 year (2.67 ± 0.28) for the CR group, and by 52.1% from baseline (4.15 ± 0.63) to 1 year (1.99 ± 0.43) for the EX group.
FIG. 2.
White blood cell levels of nucleic acid oxidative damage in the same individuals before (baseline) and after 12-month interventions of calorie restriction or exercise. Levels of RNA (A) and DNA (B) oxidative damage are shown as means ± SE. CR, calorie restriction by 20% (n = 9); EX, exercise with 20% increase in energy expenditure (n = 9). Signs mark out significant differences from baseline using paired t-tests (*p < 0.05, ***p < 0.001). Baseline levels of RNA and DNA oxidative damage did not differ significantly between CR and EX groups.
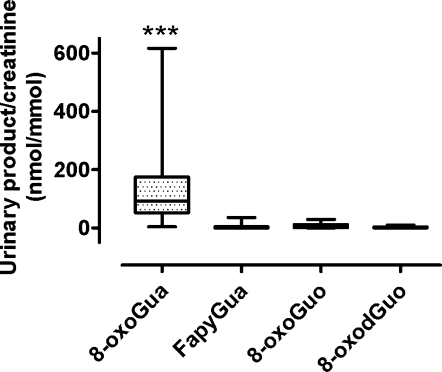
Urine levels of nucleoside oxidation products. Urinary levels for each nucleic acid oxidation product at baseline and at one-year are presented in Table 1. In contrast to the WBC data, nucleic acid oxidation products were not found to be significantly different from baseline following either 12-month intervention program. We did, however, find differences in the amount of baseline levels between the different excreted oxidized nucleic acid bases. Comparison of urinary excretion levels of the four nucleic acid products at baseline before interventions in all groups were different: baseline levels of 8-oxoGua (162 ± 33 nmol 8-oxoGua/mmol creatinine) was significantly higher (p < 0.001) than the levels of FapyGua (4.19 ± 1.4), 8-oxoGuo (6.68 ± 1.5), and 8-oxodGuo (2.37 ± 0.50) (Fig. 3).
Table 1.
Urinary Levels of RNA and DNA Oxidation Products
| |
EX (n = 15) |
CR (n = 14) |
||
|---|---|---|---|---|
| Baseline | One year | Baseline | One year | |
| Creatinine | 6.42 ± 0.89 | 6.93 ± 1.3 | 8.03 ± 2.2 | 8.83 ± 1.7 |
| FapyGua/creatinine | 4.50 ± 2.4 | 3.25 ± 1.0 | 3.86 ± 1.5 | 2.72 ± 0.74 |
| 8-oxoGua/creatinine | 127 ± 28 | 144 ± 44 | 200 ± 60 | 141 ± 34 |
| 8-oxoGua/creatinine | 6.28 ± 2.1 | 5.34 ± 1.5 | 7.12 ± 2.0 | 6.21 ± 1.7 |
| 8-oxoGua/creatinine | 2.30 ± 0.74 | 2.78 ± 0.82 | 2.44 ± 0.71 | 2.47 ± 1.1 |
Levels of FapyGua, 8-oxoGua, 8-oxoGuo, and 8-oxodGuo are expressed as nmol/mmol creatinine, creatinine alone as mM. Values are given as mean ± SE.
FIG. 3.
Comparison of urinary excretion levels of the four nucleic acid products at baseline before interventions. Data are shown as box-and-whisker plots (box covers 25th to 75th percentiles with line at median) showing a large variance between individuals in 8-oxoGua/creatinine levels and the highest levels compared to the other oxidized nucleic acids. ***Significantly different from all other products with Kruskal-Wallis and Dunn's post test (p < 0.001, n = 29).
Discussion
In this 1 year randomized trial, we compared the effects of weight loss induced by CR or EX, both producing a 20% energy deficit, on DNA and RNA oxidative damage in healthy normal weight and overweight middle-aged men and women. Our results provide evidence that negative energy balance induced through both CR and EX decrease levels of oxidative RNA and DNA damage in WBC to a similar extent. No significant changes in urinary nucleic acid oxidation levels were found. The reason for these disparate findings is not entirely clear but may be related to build-up of oxidative damages in WBC over longer time periods. Additionally, the large individual differences we observed in urinary levels of nucleic acid oxidation products makes determining statistical differences difficult. The variability was very apparent when comparing the four different nucleic acid oxidation products, with 8-oxoGua/creatinine having the highest levels and greatest variability.
This study represents a first attempt to examine the effects of energy deficits created from either prolonged CR or EX over 1 year on both oxidative RNA and DNA damage. In line with our findings, a previous 6-month study found that both a CR only (25% CR) and a CR plus EX intervention (12.5% CR plus 12.5% increase in energy expenditure through EX) reduced DNA strand breaks in human WBC, assessed through the comet assay.26 Additionally, other studies have found that oxidative DNA damage to leucocytes is reduced in physically active individuals, as compared to sedentary persons, following an acute bout of exercise.27 The reduction in oxidative DNA and RNA damage to WBC we observed may be due to a reduction in systemic inflammation and related production of reactive oxygen species (ROS)28; both CR and EX have been found to reduce circulating markers of inflammation, such as C-reactive protein (CRP) and related cytokines (e.g., interleukins).29,30 In addition to affecting systemic hormone and metabolic regulatory parameters, CR and EX may affect behavioral patterns (e.g., sleep duration and quality). Moreover, a recent theory suggest that metabolic syndromes (obesity and type 2 diabetes) are related to changes in gut microbial composition and mass, where uptake of metabolites and gram-negative bacteria released lipopolysacharide (LPS) affect systemic metabolic parameters and can increase the systemic inflammation related to ROS production.31 Both CR and EX can affect the gut microbial mass as well as composition.
Further, it has been unknown which RNA and DNA oxidation product is mainly excreted in urine as it has been debated if the initial 8-OH-8-H-Gua• radical intermediate (Fig. 1) formed after oxidant attack undergoes an oxidation (forms 8-oxoGua) or a reduction (ring opening into FapyGua).20,32 The clearly higher concentrations of 8-oxoGua than FapyGua found in human urine (Fig. 3) strongly supports an oxidation of the 8-OH-8-H-Gua• radical intermediate as the major mechanism. As the base guanine is the moiety having the lowest oxidation potential in both RNA and DNA,32 the urine suggest that 8-oxoGua is the major nucleic acid lesion formed and excreted in humans as result of oxidative damage. However, we cannot rule out that urinary 8-oxoGua stems from RNA or DNA as it could stem from both, after being recognized, removed, and excreted. Excretion of the specific RNA oxidation product 8-oxoGuo was higher than the specific DNA oxidation product 8-oxodGuo. It should also be noted that oxidative base lesions in urine may to a certain extent also stem from absorption of digested oxidized food products, from gut microbial end products, or from dead cells which could affect the results.22–24 In DNA, oxidized bases are mainly removed by base excision repair (BER), whereas the processes for removal of damaged RNA (rRNA, tRNA, mRNA, and siRNA) are largely unknown. In agreement with previous observations,33,34 we found that the urinary level of the RNA-specific oxidation product 8-oxoGuo was higher that that of DNA (8-oxodGuo) for both CR and EX groups at baseline.
The results of the present study should be interpreted in the context of its limitations. First, the generalizability of these findings is limited by our small sample size, as well as restricted body mass index range. Thus, these findings need to be replicated in future studies that utilize larger sample sizes and more diverse populations. Another weakness is the inability to completely control for food intake during the week prior to the collection of the urine samples, which may explain the variability of the oxidized nucleic acids in the urine, specifically for 8-oxoGua. The present study also had a number of strengths. This is the first study to test the effects of energy deficits created through prolonged CR versus EX on DNA and RNA oxidative damage, and the first report of RNA oxidative damage levels in WBC. Other strengths include the use of a randomized controlled trial design, comprehensive assessments of energy intake and expenditure using DLW, and the high rate of adherence to both interventions, as evidenced by the significant weight loss in both groups.
In conclusion, prolonged CR and EX interventions, both producing a 20% energy deficit, were found to decrease oxidative DNA and RNA damage in human WBC. However, significant changes in DNA and RNA oxidation products were not observed through urinary analyses, potentially due to greater individual variability on this measure. Overall, our findings suggest energy deficits created through both CR and EX reduce DNA and RNA damage to WBC, potentially by reducing systemic oxidative stress.
Acknowledgments
We would like to thank Dr. Emanuele Marzetti for his critical input into this research. This research was supported by grants from the NIH Cooperative Agreement (AG20487), NIH General Clinical Research Center (RR00036), Diabetes Research Training Center (DK20579), and NIH Clinical Nutrition Research Unit (DK56341), and from the National Institute on Aging to CL (AG17994 and AG21042), and an American Heart postdoctoral fellowship to TH (0525346B). This work was also supported by the University of Florida Institute on Aging, the Claude D. Pepper Older Americans Independence Center NIH (grant 1 P30 AG028740), and the General Clinical Research Centre (GCRC support grant M01-RR00082).
References
- 1.Gilchrest BA. Bohr VA. Aging processes, DNA damage, and repair. FASEB J. 1997;11:322–330. doi: 10.1096/fasebj.11.5.9141498. [DOI] [PubMed] [Google Scholar]
- 2.Sohal RS. Mockett RJ. Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Garcia O. Vega-Naredo I. Sierra V. Caballero B. Tomas-Zapico C. Camins A. Garcia JJ. Pallas M. Coto-Montes A. Elevated oxidative stress in the brain of senescence-accelerated mice at 5 months of age. Biogerontology. 2006;7:43–52. doi: 10.1007/s10522-005-6041-2. [DOI] [PubMed] [Google Scholar]
- 4.Vincent HK. Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006;30:400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 5.Dandona P. Mohanty P. Ghanim H. Aljada A. Browne R. Hamouda W. Prabhala A. Afzal A. Garg R. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab. 2001;86:355–362. doi: 10.1210/jcem.86.1.7150. [DOI] [PubMed] [Google Scholar]
- 6.Davi G. Guagnano MT. Ciabattoni G. Basili S. Falco A. Marinopiccoli M. Nutini M. Sensi S. Patrono C. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288:2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 7.Judge S. Leeuwenburgh C. Cardiac mitochondrial bioenergetics, oxidative stress, and aging. Am J Physiol Cell Physiol. 2007;292:C1983–1992. doi: 10.1152/ajpcell.00285.2006. [DOI] [PubMed] [Google Scholar]
- 8.Radak Z. Naito H. Kaneko T. Tahara S. Nakamoto H. Takahashi R. Cardozo-Pelaez F. Goto S. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch. 2002;445:273–278. doi: 10.1007/s00424-002-0918-6. [DOI] [PubMed] [Google Scholar]
- 9.Seo AY. Hofer T. Sung B. Judge S. Chung HY. Leeuwenburgh C. Hepatic oxidative stress during aging: effects of 8% long-term calorie restriction and lifelong exercise. Antioxid Redox Signal. 2006;8:529–538. doi: 10.1089/ars.2006.8.529. [DOI] [PubMed] [Google Scholar]
- 10.Fontana L. Klein S. Aging, adiposity, and calorie restriction. JAMA. 2007;297:986–994. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- 11.Holloszy JO. Mortality rate and longevity of food-restricted exercising male rats: a reevaluation. J Appl Physiol. 1997;82:399–403. doi: 10.1152/jappl.1997.82.2.399. [DOI] [PubMed] [Google Scholar]
- 12.Rosa EF. Takahashi S. Aboulafia J. Nouailhetas VL. Oliveira MG. Oxidative stress induced by intense and exhaustive exercise impairs murine cognitive function. J Neurophysiol. 2007;98:1820–1826. doi: 10.1152/jn.01158.2006. [DOI] [PubMed] [Google Scholar]
- 13.Venditti P. Bari A. Di Stefano L. Di Meo S. Role of mitochondria in exercise-induced oxidative stress in skeletal muscle from hyperthyroid rats. Arch Biochem Biophys. 2007;463:12–18. doi: 10.1016/j.abb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Huffman DM. Moellering DR. Grizzle WE. Stockard CR. Johnson MS. Nagy TR. Effect of exercise and calorie restriction on biomarkers of aging in mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1618–1627. doi: 10.1152/ajpregu.00890.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Racette SB. Weiss EP. Villareal DT. Arif H. Steger-May K. Schechtman KB. Fontana L. Klein S. Holloszy JO. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61:943–950. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss EP. Racette SB. Villareal DT. Fontana L. Steger-May K. Schechtman KB. Klein S. Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss EP. Racette SB. Villareal DT. Fontana L. Steger-May K. Schechtman KB. Klein S. Ehsani AA. Holloszy JO. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol. 2007;102:634–640. doi: 10.1152/japplphysiol.00853.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malayappan B. Garrett TJ. Segal M. Leeuwenburgh C. Urinary analysis of 8-oxoguanine, 8-oxoguanosine, fapy-guanine and 8-oxo-2′-deoxyguanosine by high-performance liquid chromatography-electrospray tandem mass spectrometry as a measure of oxidative stress. J Chromatogr A. 2007;1167:54–62. doi: 10.1016/j.chroma.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Cadet J. Douki T. Gasparutto D. Ravanat JL. Oxidative damage to DNA: formation, measurement and biochemical features. Mutat Res. 2003;531:5–23. doi: 10.1016/j.mrfmmm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Hofer T. Oxidation of 2′-deoxyguanosine by H2O2-ascor-bate: evidence against free OH. and thermodynamic support for two-electron reduction of H2O2. J Chem Soc Perkin Trans. 2001;2:210–213. [Google Scholar]
- 21.Hofer T. Badouard C. Bajak E. Ravanat JL. Mattsson A. Cot-greave IA. Hydrogen peroxide causes greater oxidation in cellular RNA than in DNA. Biol Chem. 2005;386:333–337. doi: 10.1515/BC.2005.040. [DOI] [PubMed] [Google Scholar]
- 22.Cooke MS. Olinski R. Loft S. Measurement and meaning of oxidatively modified DNA lesions in urine. Cancer Epidemiol Biomarkers Prev. 2008;17:3–14. doi: 10.1158/1055-9965.EPI-07-0751. [DOI] [PubMed] [Google Scholar]
- 23.Lee CY. Isaac HB. Wang H. Huang SH. Long LH. Jenner AM. Kelly RP. Halliwell B. Cautions in the use of biomarkers of oxidative damage; the vascular and antioxidant effects of dark soy sauce in humans. Biochem Biophys Res Commun. 2006;344:906–911. doi: 10.1016/j.bbrc.2006.03.217. [DOI] [PubMed] [Google Scholar]
- 24.Rozalski R. Siomek A. Gackowski D. Foksinski M. Gran C. Klungland A. Olinski R. Diet is not responsible for the presence of several oxidatively damaged DNA lesions in mouse urine. Free Radic Res. 2004;38:1201–1205. doi: 10.1080/10715760400017350. [DOI] [PubMed] [Google Scholar]
- 25.Hofer T. Seo AY. Prudencio M. Leeuwenburgh C. A method to determine RNA and DNA oxidation simultaneously by HPLC-ECD: greater RNA than DNA oxidation in rat liver after doxorubicin administration. Biol Chem. 2006;387:103–111. doi: 10.1515/BC.2006.014. [DOI] [PubMed] [Google Scholar]
- 26.Heilbronn LK. de Jonge L. Frisard MI. DeLany JP. Larson-Meyer DE. Rood J. Nguyen T. Martin CK. Volaufova J. Most MM. Greenway FL. Smith SR. Deutsch WA. Williamson DA. Ravussin E. Effect of 6-month calorie restriction on bio-markers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asami S. Hirano T. Yamaguchi R. Itoh H. Kasai H. Reduction of 8-hydroxyguanine in human leukocyte DNA by physical exercise. Free Radic Res. 1998;29:581–584. doi: 10.1080/10715769800300621. [DOI] [PubMed] [Google Scholar]
- 28.Chung HY. Sung B. Jung KJ. Zou Y. Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 29.Fontana L. Meyer TE. Klein S. Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer TE. Kovacs SJ. Ehsani AA. Klein S. Holloszy JO. Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 31.Cani PD. Delzenne NM. Gut microflora as a target for energy and metabolic homeostasis. Curr Opin Clin Nutr Metab Care. 2007;10:729–734. doi: 10.1097/MCO.0b013e3282efdebb. [DOI] [PubMed] [Google Scholar]
- 32.Steenken S. Jovanovic SV. How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J Am Chem Soc. 1997;119:617–618. [Google Scholar]
- 33.Park EM. Shigenaga MK. Degan P. Korn TS. Kitzler JW. Wehr CM. Kolachana P. Ames BN. Assay of excised oxidative DNA lesions: isolation of 8-oxoguanine and its nucleoside derivatives from biological fluids with a monoclonal antibody column. Proc Natl Acad Sci USA. 1992;89:3375–3379. doi: 10.1073/pnas.89.8.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weimann A. Belling D. Poulsen HE. Quantification of 8-oxoguanine and guanine as the nucleobase, nucleoside and deoxynucleoside forms in human urine by high-performance liquid chromatography-electrospray tandem mass spectrometry. Nucleic Acids Res. 2002;30:E7. doi: 10.1093/nar/30.2.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]