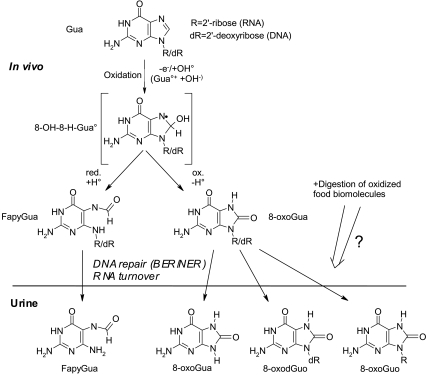
FIG. 1.
Guanine oxidation reaction scheme with structures of nucleosides and bases analyzed using HPLC-ECD and HPLC-MS/MS. The reaction mechanism of guanine (Gua) with oxidizing agents, such as one-electron oxidants (produces a Gua•+ cation that can react with a hydroxyl anion, OH−) and the hydroxyl radical (OH•), forms an unstable intermediate radical (8-OH-H-Gua•) upon reaction at the exposed C-8 position.19,20 The reduction reaction (+e− +H+ (= +H•)) generates an 8-OH-H-Gua• ring opening to form FapyGua, whereas upon oxidation (-H•) 8-oxoGua is formed.19,20 Base and nucleotide excision repair (BER/NER) systems constantly proof DNA for mutagenic oxidation products to be removed from the cells and excreted into the plasma and filtered by the kidney. How oxidation products in RNA are removed is presently unclear, but is believed to occur by yet largely undefined mechanisms turning over damaged RNA.21 8-oxoGua and FapyGua can be excreted as free bases or attached to the sugar moieties present in RNA and DNA (R = ribose, dR = 2′-deoxyribose). FapyGua forms several isomers when attached to a sugar moiety and was measured as free base only. Urinary nucleoside oxidation products may to a minor extent also be absorbed from digested food or gut microbial end products, or stem from dead cells.22–24