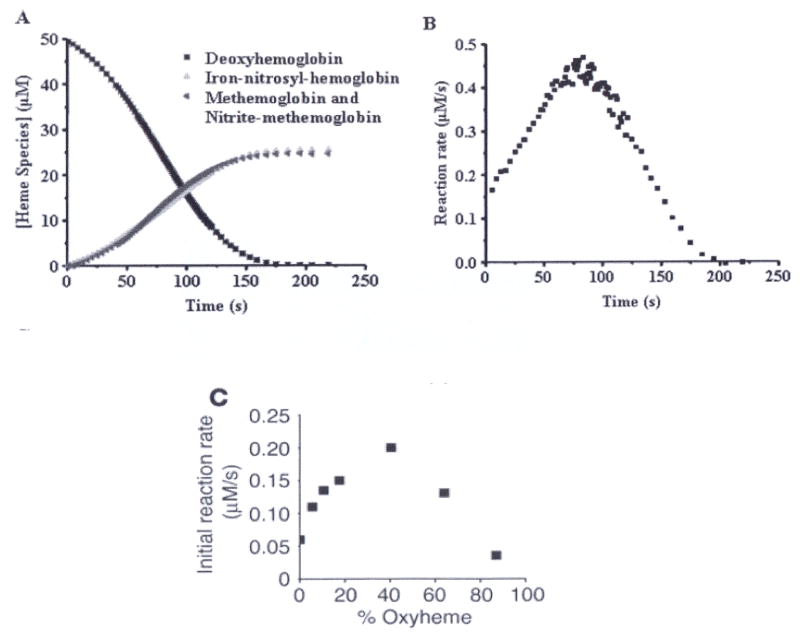
Figure 1.
Kinetics of the reaction between human deoxyHb (50 μM) and nitrite (10 mM) at pH 7.4 and 37° C. (A) UV/VIS absorption spectra were deconvoluted to determine the percentage of each species as a function of time. DeoxyHb is observed to form equal amounts of metHb and iron-nitrosyl-Hb. Deviation from first order behavior is evident in the curve for decay of deoxyHb, having a sigmoidal shape. (B) The instantaneous rate of the reaction shown in panel A where the negative of the slope of the decay curve for deoxyHb is plotted as a function of time. (C) Nitrite (10 mM) was reacted with Hb (50 μM) at various oxygen tensions. The initial rate of the reaction is plotted. (A, B from (114), C from (111) with permission).