Figure 4.
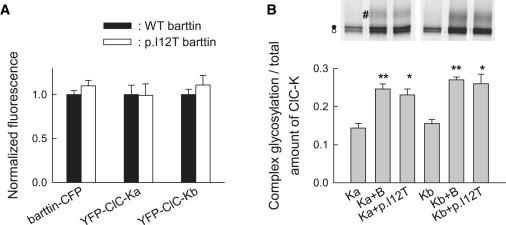
p.I12T Neither Affects the Number of Expressed ClC-K Channels nor the Glycosylation Status
(A) Fluorescent protein expression levels from HEK293T cells coexpressing WT or p.I12T barttin-CFP with YFP-ClC-Ka or YFP-ClC-Kb. For each experiment, SDS-PAGE gels (6%–15%) were prepared from the lysates of whole dishes of comparable cell number and transfection efficiency. Fluorescence was quantified with the ImageQuant software and normalized to corresponding fluorescence levels from cells coexpressing WT barttin. Data represent means ± SEM from eight or nine measurements.
(B) SDS-PAGE gel (6%–15%) of YFP-ClC-Ka or YFP-ClC-Kb either transfected alone or cotransfected with WT or mutant barttin in MDCKII cells shows complex-glycosylated (#) as well as core-glycosylated (●) or not glycosylated (◯) protein. The lower panel shows ratio of complex-glycosylated to total YFP-ClC-Ka and YFP-ClC-Kb fluorescence from cells coexpressing WT or mutant barttin. Mean ± SEM is from three or more experiments. Asterisks indicate levels of significance (∗∗p < 0.01 or ∗p < 0.05) for comparison with cells without barttin. Glycosylation levels were not different for cells expressing WT or p.I12T barttin.
