Figure 3.
Concentration and Activity Levels of MMP9 and MMP13 in MAD Patients
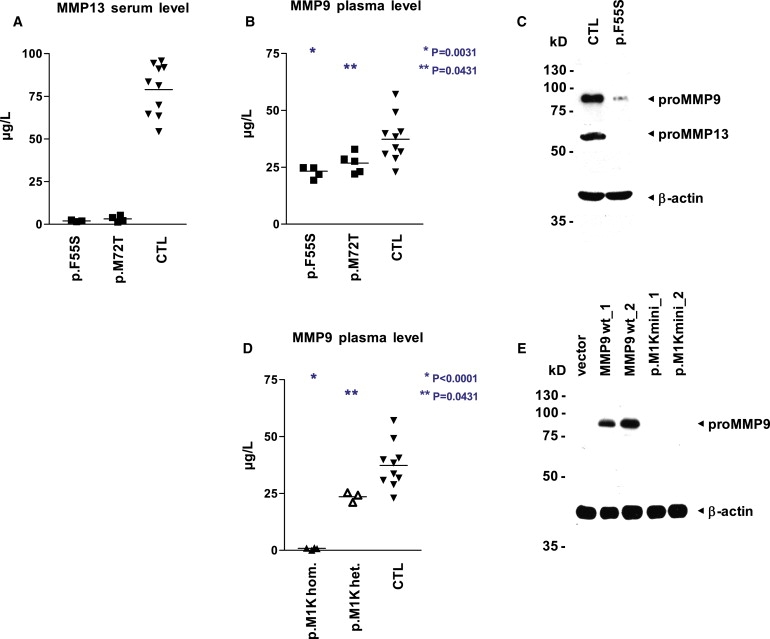
(A and B) MMP13 activity is not detectable in the serum (A), and MMP9 activity is reduced in the plasma (B), of patients with dominant MAD (MMP13 p.F55S, n = 3; MMP13 p.M72T, n = 3) in comparison to unaffected controls (n = 10). Specific MMP activity was quantified via a fluorogenic peptide substrate cleavage assay. Samples were measured in quadruplicates, and p values were obtained with a two-tailed Student's t test for the comparison of means (horizontal line).
(C) Both proMMP13 and proMMP9 are strongly reduced in immunoblot analysis of whole-cell lysates from early-passage fibroblasts of patient 1 with dominant MAD (MMP13 p.F55S) as compared with an age-matched unaffected control; equal loading is demonstrated by reprobing with an antibody directed against β-actin.
(D) The plasma of patients with recessive MAD lacks MMP9 activity (MMP9 c.21T>A, p.M1K, n = 2), and heterozygous carriers (n = 2) show a significant reduction in comparison to unaffected controls (n = 10). MMP9 activity was quantified via a fluorogenic peptide substrate cleavage assay. Samples were measured in triplicates and p values were obtained with a two-tailed Student's t test for the comparison of means (horizontal line).
(E) No lower-sized bands indicative of initiation at downstream start codons are visible in immunoblot analysis of whole-cell lysates from fibroblasts transfected with minigene constructs bearing the recessive-MAD-associated mutation MMP9 c.21T>A, p.M1K. In wild-type constructs, proMMP9 is readily detectable with an antibody directed against the carboxy terminus. Transfection efficiency was similar, as monitored by fluorescence microscopy of EGFP expressed from an internal ribosome entry site downstream of MMP9; the promoter region includes 1644 bp (construct_1) and 762 bp (construct_2) upstream of the MMP9 start codon.
