Fig. 1.
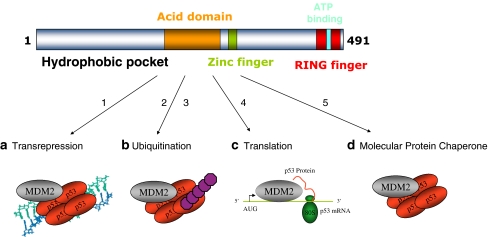
Four distinct modes of MDM2 function. The top panel highlights the domain structure of MDM2, including the hydrophobic pocket in the N-terminus [20], the acidic central domain [55], a zinc-binding motif [56], the RING domain [18], and ATP-binding site imbedded within the RING domain [35]. MDM2 was identified as a protein that regulates the p53 tumor suppressor via (1) inhibition of p53-dependent transcription [26, 43]. Germline mutations in the mdm2 promoter that result in MDM2 over-production result in enhanced MDM2 protein in chromatin fractions, which mediates suppression of p53 function as a transcription factor [2, 52]. The second function identified for MDM2 involved its ability to (2, 3) catalyze p53 degradation through a ubiquitin-dependent pathway [19] [15]. This ubiquitination function of MDM2 also involves ubiquitination and degradation of ribosomal proteins that can normally function to stimulate p53 protein synthesis [31]. According to the dual-site mechanism of MDM2-mediated ubiquitination of p53 [50], two structural changes can enhance p53 ubiquitination by MDM2: one structural change involves (2) destabilizing missense mutations in p53 that unfolds the p53 tetramer and that can expose the ubiquitination signal in the DNA-binding domain [38], thus enhancing p53 ubiquitination in cells [39]; the second structural change involves (3) the lid of MDM2 whose phospho-mimetic mutation increases MDM2-mediated ubiquitination of p53 tetramers by stabilizing the MDM2:p53 complex (this study). Small molecules that bind to the N-terminal hydrophobic pocket of MDM2 (Nutlins) can activate p53 [47] presumably by inhibiting the chromatin-bound pool of MDM2 since Nutlins cannot inhibit MDM2 function as an E3 ubiquitin ligase [50]. Rather, to date, peptide mimetics that bind to the MDM2 acidic domain provide a lead for inhibiting MDM2 function as a E3 ubiquitin ligase [50]. Additional research has highlighted novel molecular functions of MDM2. A cellular function was assigned to the RNA-binding activity of MDM2 [12, 21, 24], which involves (4) an interaction of MDM2 with p53 mRNA at ribosomes to stimulate p53 protein synthesis [7]. A function was also assigned to the ATP-binding motif of MDM2 [35], which involves (5) the ability of MDM2 to exhibit the property of a molecular chaperone by catalyzing the ATP-dependent folding of the p53 tetramer to enhance p53 function as a transcription factor [41, 51]