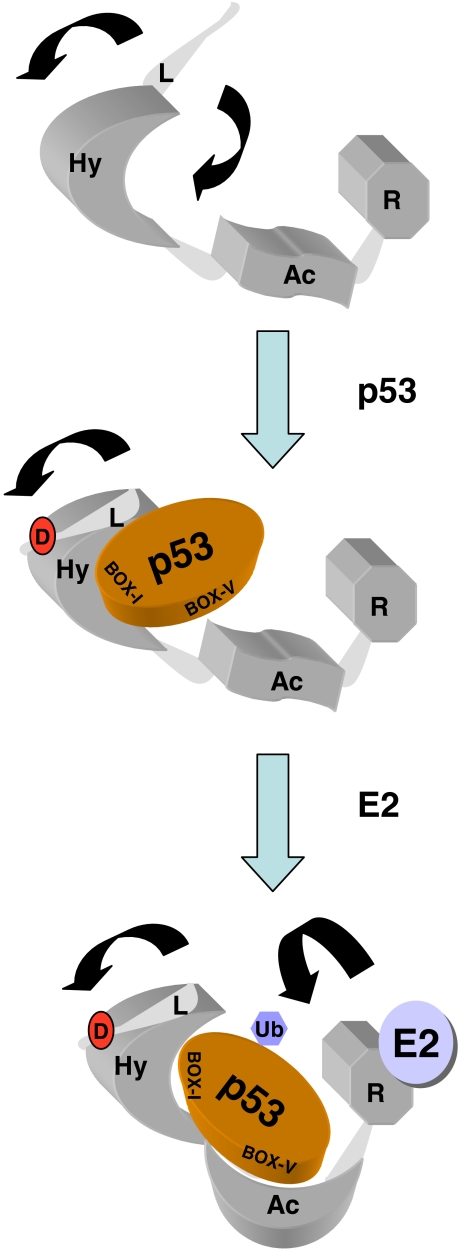
Fig. 10.
Effects of the MDM2 lid on allosteric regulation of its E3 ubiquitin ligase function. (a) MDM2 is composed of three key functional domains, including the hydrophobic pocket in the N-terminus (Hy), the acidic domain in the central portion of MDM2 (Ac), and the C-terminal RING domain (R). The N-terminus also has a flexible and unstructured pseudo-substrate motif (L), which is in equilibrium over the hydrophobic pocket (like a lid) or outwith the pocket (arrows). (b) Upon generation of the phospho-mimetic mutation in the pseudo-substrate motif, the flexible lid chain is stabilized in an equilibrium outwith the hydrophobic pocket, allowing enhanced binding to p53 at the BOX-I motif. (c) A conformational change ensues, which stabilizes the interaction between the Ac domain of MDM2 and the BOX-V motif in the DNA-binding domain of p53. This allows the E2-mediated ubiquitin transfer to the p53 substrate. The mechanisms whereby the E3:E2 interaction drives p53 substrate recognition and ubiquitin transfer is not defined nor is it known whether interactions between MDM2 and p53 occur intra or intermolecularly within the p53 tetramer. The model does not incorporate the p53 tetrameric structure since it is not known if MDM2 docking to p53 tetramers is an intra- or intermolecular mechanism