Abstract
AIM: To assess the clinical value of contrast-enhanced intraoperative ultrasound (CE-IOUS) as a novel tool in partial hepatectomy for cirrhotic patients with hepatocellular carcinoma (HCC).
METHODS: From January 2007 to September 2007, a total of 20 consecutive cirrhotic patients with HCC scheduled to undergo partial hepatectomy were studied. Preoperative contrast enhanced computer tomography (CT) and/or magnetic resonance (MR) scans were performed within 1-2 wk before operation. Intraoperative ultrasound (IOUS) and CE-IOUS were carried out after mobilization of the liver. Lesions on precontrast and postcontrast scans were counted and mapped. CE-IOUS was performed with intravenous injection of ultrasound contrast agents SonoVue (Bracco Imaging, Milan, Italy). Arterial, portal and late phases of contrast enhancement were recorded and analyzed. Nodules showing arterial phase hyper-enhancing and/or hypo-enhancing in late parenchymal phase were considered malignant and removed surgically. Ultrasound-guided biopsy and ethanol ablation would be an option if the nodule could not be removed surgically. Newly detected nodules on IOUS showing iso-enhancement in both arterial and late phases were considered benign. These nodules were either removed surgically if they were close to the main lesion or followed by examinations of alpha-fetoprotein (AFP) level and ultrasound and/or CT/MR every 3 mo.
RESULTS: IOUS found 41 nodules in total, among which 17 (41.46%) were newly detected compared to preoperative imaging. Thirty-three nodules were diagnosed malignant by CE-IOUS, including one missed by IOUS. The sensitivity and specificity of CE-IOUS on detecting HCC nodules are 100% (33/33 and 100% (9/9), respectively. Nine nodules were considered benign by CE-IOUS, four was confirmed at histology and five by follow-up. CE-IOUS changed the surgical strategy in 35% (7/20) of patients and avoid unnecessary intervention in 30% (6/20) of patients.
CONCLUSION: CE-IOUS is a useful means to charac-terize the nodules detected by IOUS in cirrhotic liver, to find isoechoic HCC nodules which can not be shown on IOUS and to improve the accuracy of conventional IOUS, thus it can be used as an essential tool in the surgical treatment of cirrhotic patients with HCC.
Keywords: Cirrhosis, Liver neoplasms, Intraoperative ultrasound, Microbubble contrast agent, Hepatectomy
INTRODUCTION
Intraoperative ultrasound (IOUS) is an important tool for surgical treatment of liver tumors[1,2], and particularly for hepatocellular carcinoma (HCC). It has been shown that IOUS is the most accurate diagnostic technique for detecting focal liver lesions (FLL) and has a great impact on the surgical approach to liver tumors[3–7]. However, in cirrhotic patients with HCC, not all nodules detected by IOUS are neoplastic[8]. Compared with CT and magnetic resonance (MR) imaging, IOUS has better spatial resolution with high frequency probe[9], however, it can not provide information about tumor vascularity and tissue microcirculation. How to differentiate small HCC from the nodules detected by IOUS poses a big challenge both for surgeons and ultrasonic doctors. The application of intravenous ultrasound contrast agents during transcutaneous ultrasonography of the liver has shown to improve nodule characterization in comparison with unenhanced ultrasound[10–14]. Therefore, we investigated if the application of contrast-enhanced ultrasound examination intraoperatively could solve the aforementioned deficiencies of IOUS during liver exploration.
MATERIALS AND METHODS
This study was conducted under the approval of the Committee of Ethics of West China Hospital and informed consents of all the patients were obtained. From January 2007 to September 2007, a total of 20 consecutive cirrhotic patients with HCC scheduled to undergo partial liver resection were studied. They included 15 men and 5 women, aged 28-68 (mean 50) years. Preoperative contrast enhanced CT and/or MR scans were performed within 1-2 wk of operation. Contrast enhanced CT scans were obtained with multi-detector CT scanners (Somatom Sensation 64, Siemens Medical Solutions, Erlangen, Germany). The patients received 120-150 mL iopromide (Ultravist 300 or 370; Schering, Berlin, Germany) at a rate of 3 mL/s. CT was performed during the arterial phase using a 25-35 s delay, portal venous phase using a 70-75 s delay, and equilibrium phase using a 3 min delay after i.v. administration with 7-mm section thickness. Contrast-enhanced MRI examinations were performed with a 1.5 T imaging system (Gyroscan Intera, Philips Medical Systems Best, Netherlands), using a breathhold 3D gradient echo sequence with fat saturation sequence, following an iv bolus of 0.1 mmol gadobenate dimeglumine (MultiHance, Bracco SpA, Milan, Italy) per kg of body weight at a rate of 2 mL/s. Data was acquired in the hepatic arterial, portal venous, and equilibrium phases. CT was performed in 15 patients and MRI in two and both in three patients. The number of the lesions was recorded.
Intraoperative imaging techniques
We used VIVID4 unit (GE, USA) with an I-shaped 10-4 MHz intraoperative probe for IOUS scans. After mobilization of the liver, IOUS was performed to search for nodules and suspected lesions were counted and mapped. CE-IOUS was carried out both for lesion characterization and new nodule detection. Considering no specific intraoperative probe is available for contrast study, we used IU22 unit (Philips, USA) equipped with a 5-2 MHz convex transducer and a 9-3 MHz linear transducer instead. Both of the probes have the capacity for contrast enhanced ultrasound studies. The contrast agent was SonoVue (Bracco Imaging, Milan, Italy) which consists of sulphur hexafluoride microbubbles stabilized by a phospholipid shell; 4.8 mL of SonoVue per exploration was injected intravenously through a peripheral vein. A low mechanical index (MI < 0.1) mode was used. All phases of contrast enhancement, including arterial (10-20 s to 25-35 s after injection), portal (30-45 s to 120 s) and late parenchymal (> 120 s) phases were recorded and analyzed[13]. HCC is characterized by arterial phase hyper-enhancing and wash out of microbubbles during the portal and late phase, while benign solid lesions are characterized by persistence of contrast enhancement during the portal and late phase[15].
Nodules showing arterial hyper-enhancement and/or hypo-enhancement in late parenchymal phase were removed surgically. Ultrasound-guided biopsy and ethanol ablation would be an alteration if the nodule can not be removed surgically. Nodules depicting isoenhancement both in arterial and late parenchymal phases were considered benign and removed only in cases located close to the main lesion and the others were followed by examinations of alpha-fetoprotein (AFP) level and ultrasound and/or spiral CT every 3 mo.
RESULTS
The number of nodules detected by different imaging methods is summarized in Table 1. The sensitivity and specificity of CE-IOUS in detecting HCC nodules is 100% (33/33) and 100% (9/9), respectively. IOUS and CE-IOUS confirmed the existence of all the 24 nodules found preoperatively. Among the 24 lesions, which were proved to be HCC at histology, 23 nodules demonstrated arterial phase hyper-enhancement and late phase hypo-enhancement (Figures 1 and 2). One nodule had no contrast agent uptake both in arterial and late parenchymal phases (Figure 3). Seventeen nodules were newly detected by IOUS (Figure 4). Among the 17 IOUS newly detected nodules, 8 were hyper-enhanced in arterial phase and hypo-enhanced in late phase on CE-IOUS and proved to be malignant at histology. The other 9 nodules illustrated isoenhancement in both arterial and late parenchymal phases (Figure 5). Four of them were removed because they were located close to the main lesions and these lesions were dysplastic nodules in histology. Five nodules were not removed and follow-up ultrasound and/or CT showed no sign of malignancy with normal AFP level after 6-15 mo. One isoehoic HCC nodule was missed by IOUS, but showed a typical contrast agent washout pattern on CE-IOUS late parenchymal phase (Figure 6).
Table 1.
HCC nodules detected at preoperative imaging, IOUS and CE-IOUS
| Patient no. |
Number of HCC nodules |
||
| Preoperative imaging | IOUS | CE-IOUS | |
| 1 | 1 | 3 | 1 |
| 2 | 2 | 4 | 2 |
| 3 | 1 | 3 | 2 |
| 4 | 2 | 2 | 2 |
| 5 | 1 | 1 | 1 |
| 6 | 1 | 1 | 1 |
| 7 | 1 | 1 | 1 |
| 8 | 1 | 2 | 1 |
| 9 | 1 | 3 | 3 |
| 10 | 1 | 2 | 1 |
| 11 | 1 | 1 | 1 |
| 12 | 1 | 1 | 1 |
| 13 | 1 | 1 | 1 |
| 14 | 2 | 2 | 2 |
| 15 | 1 | 2 | 2 |
| 16 | 2 | 5 | 4 |
| 17 | 1 | 1 | 1 |
| 18 | 1 | 3 | 2 |
| 19 | 1 | 2 | 2 |
| 20 | 1 | 1 | 2 |
| Total | 24 | 41 | 33 |
Figure 1.
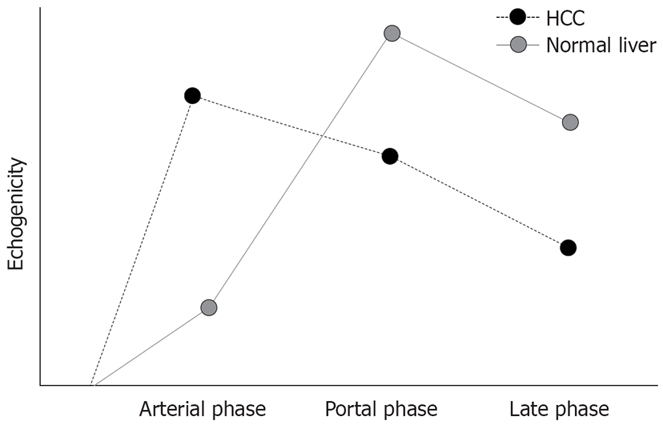
It illustrates the enhancing pattern of HCC and normal liver parenchyma. With more arterial supply, HCC appears hyperechoic in the arterial phase and the lesion is slightly and then clearly hypoechoic, and relative to the surrounding parenchyma in the portal and late phases with the washout of contrast agents.
Figure 2.
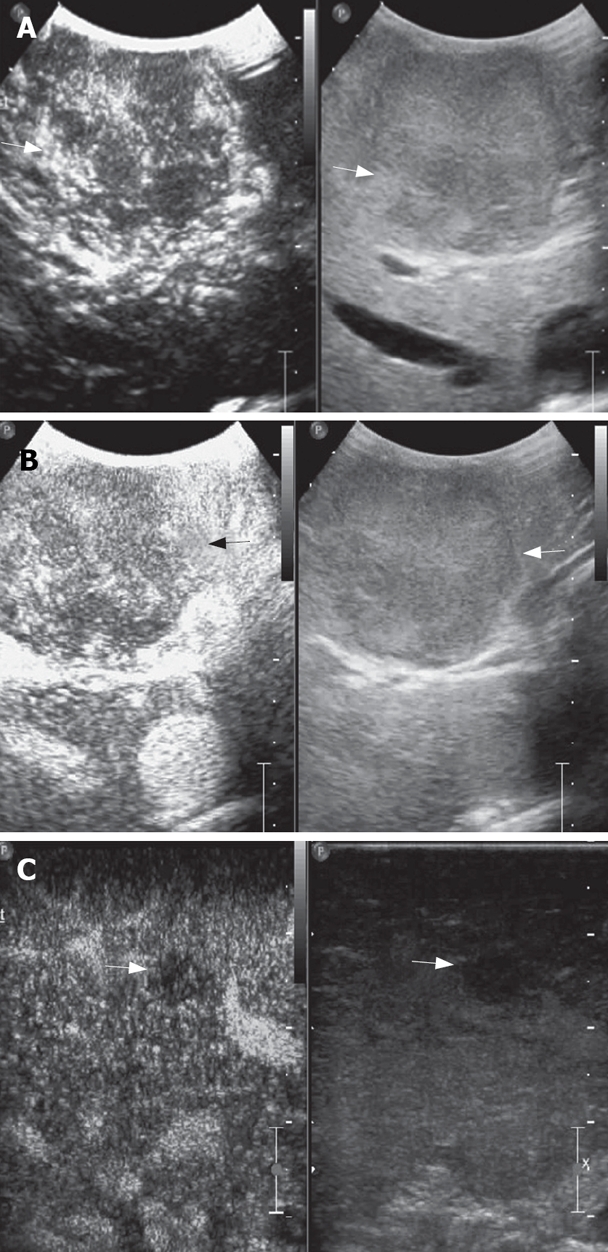
A mosaic nodule in a cirrhotic liver at IOUS. A: At arterial phase, the nodule shows early enhancement (arrows); B: The nodule demonstrates contrast agent wash-out in late phase: it was a typical appearance of HCC and proved malignant at histology (arrows); C: Another hypoechoic nodule at IOUS was hypoenhanced in late phase: it was considered intrahepatic metastasis and confirmed malignant at histology (arrows).
Figure 3.
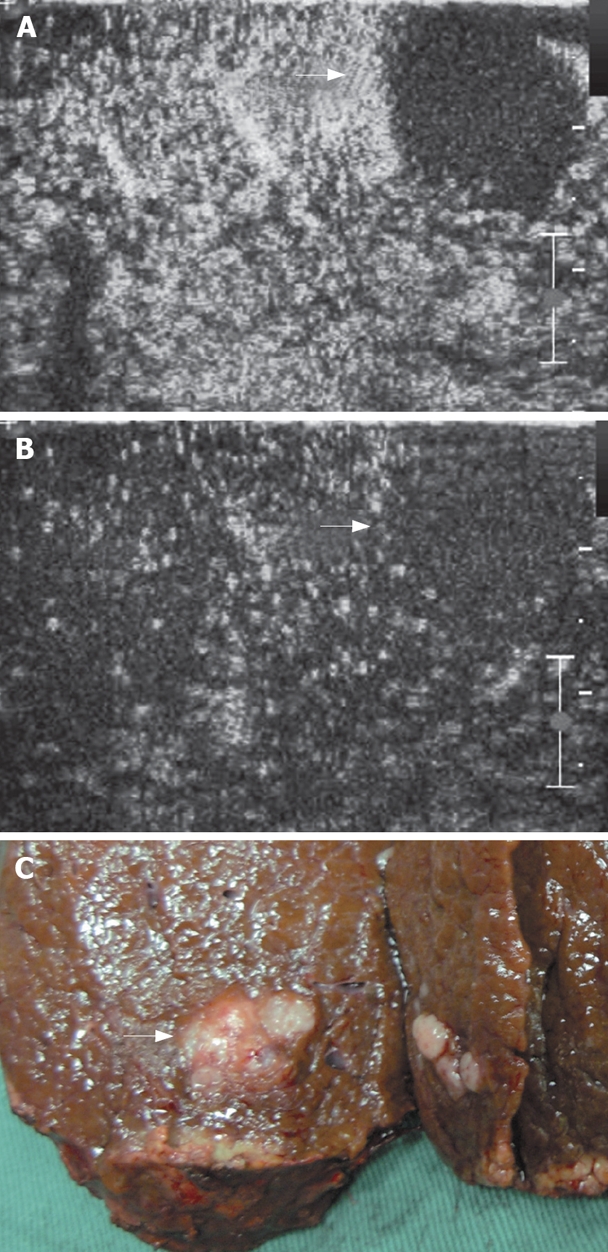
A mosaic nodule in a cirrhotic liver at IOUS. A: The nodule shows no contrast agent uptake in arterial phase (arrows); B: The nodule shows no contrast agent uptake in late parenchymal phase (arrows); C: Specimen of the mass after resection proved to be HCC at histology.
Figure 4.
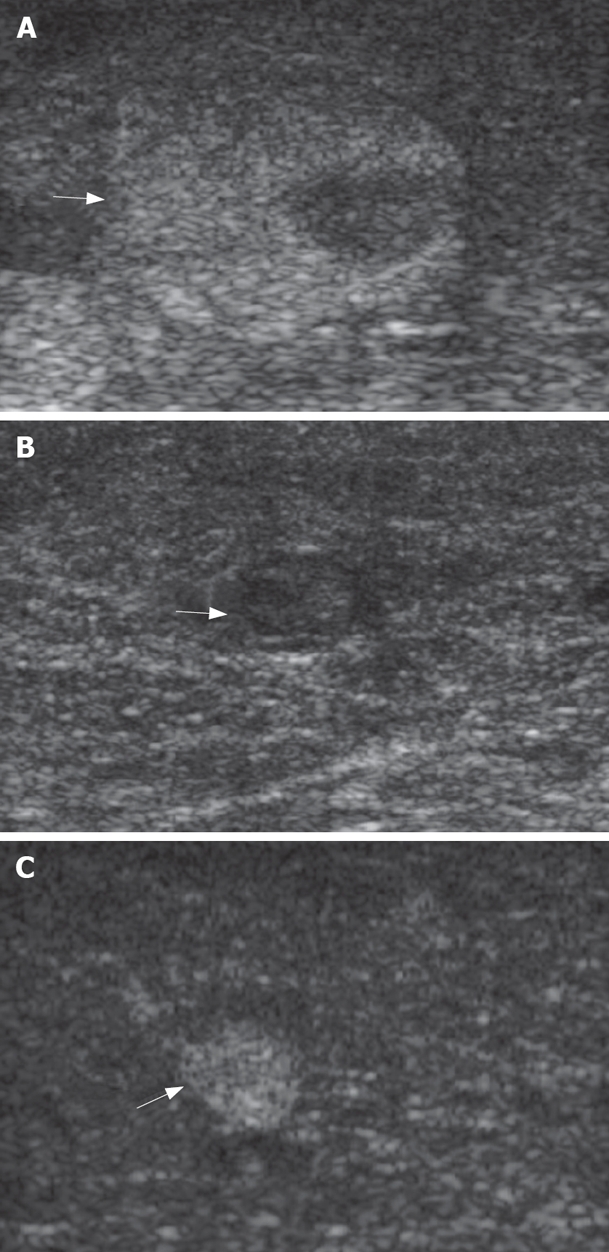
Appearance of nodules in a cirrhotic liver at IOUS. A: Mosaic pattern nodule (arrows); B: Hypoechoic nodule (arrows); C: Hyperechoic nodule (arrows).
Figure 5.
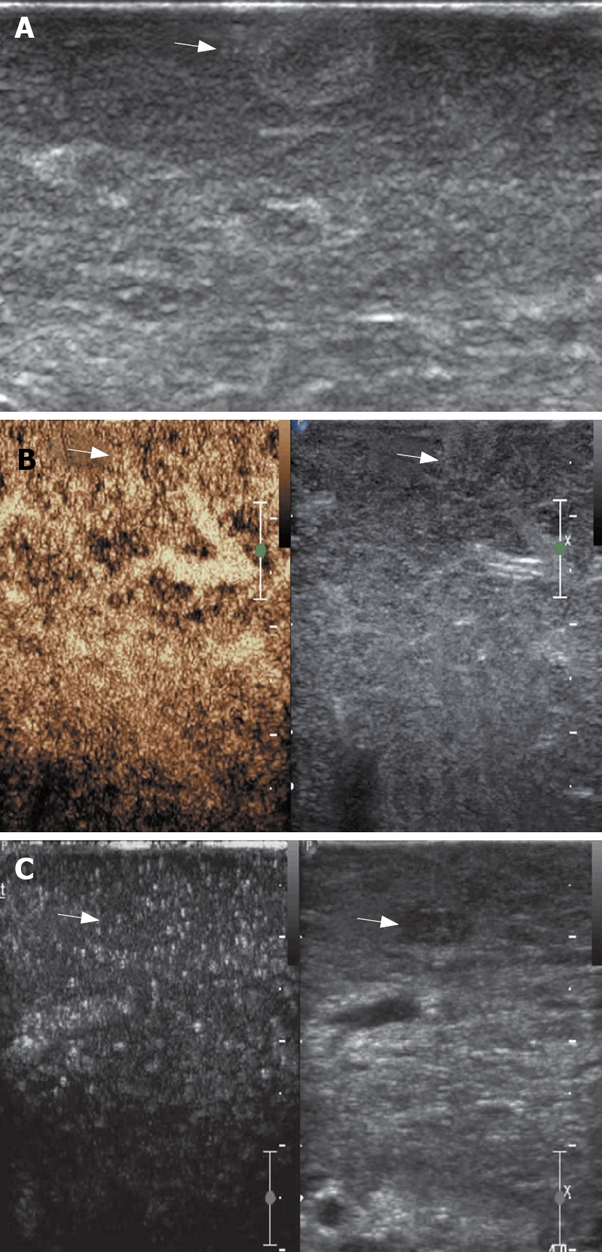
A: A hyperechoic nodule detected at IOUS in cirrhotic liver; B: The nodule shows no arterial enhancement and late parenchymal phase wash-out; C: A hypoechoic nodule detected at IOUS in cirrhotic liver, and it shows the same enhancing pattern with the surrounding parenchyma. These two nodules were considered dysplastic and the diagnosis was confirmed by pathologic examination.
Figure 6.
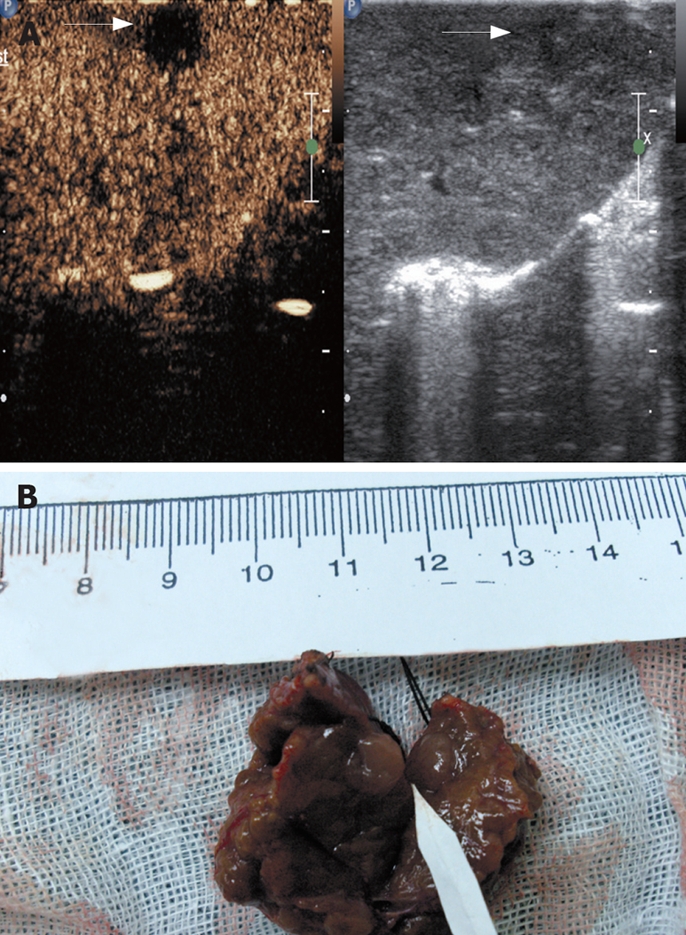
A: An isoechoic nodule measuring 7 mm was missed at IOUS but appeared clearly at late phase of CE-IOUS with contrast agent wash-out; B: Specimen of the nodule after resection proved to be HCC at histology.
CE-IOUS changed the surgical strategy in 35% (7/20) of patients, including additional tumor enucleation in five and ultrasound guided ethanol injection in two patients. CE-IOUS also avoided unnecessary intervention in 30% (6/20) of patients who otherwise need additional nodule enucleation or extended hepatectomy according to the IOUS results.
DISCUSSION
Radicality and preservation as much remnant liver parenchyma as possible are a goal for hepatic resection for cirrhotic patients with HCC. Since it is not uncommon that multiple nodules exist in cirrhotic liver with HCC, differential diagnosis of such nodules is critical, because the operation strategy may have to be changed if another HCC lesion is found. Utilizing high frequency probe, compared with CT and MRI, IOUS has better spatial resolution and it is regarded as the most accurate diagnostic method for detecting FLL. In our study, besides the 24 nodules which were already detected preoperatively, IOUS found 17 new nodules in 50% (10/20) of patients. However, new nodules lack specific IOUS findings for HCC. Some studies showed that nodules with a mosaic ultrasound pattern are malignant in 84% of cases; about 24%-30% of hypoechoic nodules, and 0%-18% of hyperechoic nodules are malignant[16]. In our study, only 47.06% (8/17) of the IOUS newly detected nodules were confirmed to be HCC at histology. Patients would be at higher risk for postoperative complications if all the nodules detected by IOUS were removed. So it is urgent that new ways are developed to increase the accuracy of IOUS.
Studies suggest that liver ultrasound performed with microbubble contrast agents has led to improved characterization and detection of FLL since it provides information about tumor vascularity and tissue microcirculation comparable to that provided by contrast-enhanced CT and MR imaging, with the added benefit of real-time imaging[17,18]. Different enhancing patterns to differentiate FLL have been described[19,20], for example, typical HCC shows arterial phase hyperenhancement and wash-out of contrast agent in portal and late parenchymal phase. The late phase is useful to determine the benign or malignant nature of a lesion while the arterial phase helps predict its histology[21–25]. About 86%-93% of benign lesions retain the contrast in the late phase, while 78%-98% of the malignant ones demonstrate wash-out of the contrast[21,22].
In our study, CE-IOUS confirmed the malignancy of the 24 HCC nodules detected preoperatively and showed the vascularity and microcirculation of the IOUS newly detected nodules. In total, 96.97% (32/33) of the HCC nodules depicted arterial phase hyperenhancement and 100% (33/33) showed late phase hypoenhancement, while 100% (9/9) of dysplastic nodules illustrated isoenhancement in both arterial phase and late phase.
CE-IOUS can also help find isoechoic HCC nodules. An isoechoic nodule measuring 7 mm which was missed at IOUS showed a typical contrast agent wash-out in late phase at CE-IOUS. Its malignancy was confirmed at histology. This phenomenon highlights the importance of scanning of the whole cirrhotic liver in late phase of CE-IOUS to detect occult lesions at IOUS.
It is known that IOUS affects surgical strategy in 20%-43.8% of patients with liver tumors[26–28]. CE-IOUS findings further demonstrate the benign or malignant nature of the lesions detected by IOUS. Since the duration of late parenchymal phase is much longer than arterial phase, a comprehensive assessment of the whole liver parenchyma which can not be accomplished in arterial phase is required in late phase. Generally, resection of the hypo-enhanced nodules in late phase on CE-IOUS is recommended. In our study, CE-IOUS changed the surgical strategy in 35% (7/20) of patients and avoided unnecessary intervention in 30% (6/20) of patients. That is to say CE-IOUS makes IOUS more accurate, thus enhancing the impact of this technique on operative decision-making for liver tumors.
There is still atypical enhancing mode of HCC. One nodule with a diameter of 2 cm which was enhanced in preoperative CT, mosaic at IOUS and hard on palpation showed no contrast agent uptake in both arterial and late phases. It was proved to be HCC at histology. This atypical pattern of enhancement of HCC nodule at CE-IOUS, when compared with that at spiral CT, supposes that some tumor vascular architecture may permit the spread of CT contrast agent while prevent that of ultrasound contrast agent[29]. However, this needs further studies to find any pathological correlations.
In our experience, high frequency probe (L9-3) is more suitable for CE-IOUS study of superficial lesions because of its high resolution in the near field. But for lesions deeper than 3 cm, lower frequency probe (C5-2) is recommended for better penetration and echo-enhancement. The respiratory tract pressure increased during general anesthesia and the scanning is performed directly on the liver surface, bubble destruction thus increased[30]. Therefore 4.8 mL SonoVue per exploration is recommended for CE-IOUS.
In conclusion, though only preliminary experience is available, CE-IOUS appears to be an exciting and promising new application. It helps differentiate nodules detected by IOUS in cirrhotic liver and find isoechoic nodules which can not be shown on IOUS, and then improves the accuracy of conventional IOUS. Considering the small number of patients, more studies are needed for further evaluation of this new technique.
COMMENTS
Background
Intraoperative ultrasound (IOUS) is an important tool for liver tumors, but it has some drawbacks such as lack of specificity to differentiate cirrhotic nodules from small malignant nodules. Contrast-enhanced ultrasound was shown with improved nodule characterization by recent studies. Therefore, authors investigated if the application of contrast-enhanced ultrasound intraoperatively could solve the aforementioned deficiencies of IOUS.
Innovations and breakthroughs
The application of CE-IOUS improves nodule characterization. It helps surgeons to eradicate malignant nodules and avoid unnecessary intervention of benign ones.
Applications
CE-IOUS raised the accuracy of conventional IOUS, thus improving the management of nodules detected in liver surgeries.
Terminology
To apply ultrasound examination during surgeries, IOUS is considered an essential tool for detecting focal liver lesions (FLL) and has a great impact on the surgical approach to liver tumors. Besides the merits of IOUS, CE-IOUS can display the tumor vascularity and tissue microcirculation using intravenous ultrasound contrast agents, thus helping differentiate malignant nodules from benign ones.
Peer review
The research is quite interesting and the results are relevant to improve the quality of hepatic surgery, mainly in cirrhotic patients. The role of contrast-enhanced ultrasound in the study of hepatocellular carcinoma has already been studied in the preoperative setting. However few studies have focused on its intraoperative role, and thus this report is quite innovative.
Peer reviewers: Toru Ikegami, MD, Department of Surgery and Science, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan; Laura Llado, PhD, Liver Transplant Unit, Department of Surgery, Hospital U Bellvitge, Feixa Llarga S/N, Hospitalet LL (Barcelona) 08907, Spain
S- Editor Li DL L- Editor Ma JY E- Editor Yin DH
References
- 1.Torzilli G, Makuuchi M. Intraoperative ultrasonography in liver cancer. Surg Oncol Clin N Am. 2003;12:91–103. doi: 10.1016/s1055-3207(02)00084-4. [DOI] [PubMed] [Google Scholar]
- 2.Torzilli G, Leoni P, Gendarini A, Calliada F, Olivari N, Makuuchi M. Ultrasound-guided liver resections for hepatocellular carcinoma. Hepatogastroenterology. 2002;49:21–27. [PubMed] [Google Scholar]
- 3.Conlon R, Jacobs M, Dasgupta D, Lodge JP. The value of intraoperative ultrasound during hepatic resection compared with improved preoperative magnetic resonance imaging. Eur J Ultrasound. 2003;16:211–216. doi: 10.1016/s0929-8266(02)00075-7. [DOI] [PubMed] [Google Scholar]
- 4.Cerwenka H. Intraoperative ultrasonography during planned liver resections remains an important surgical tool. Surg Endosc. 2008;22:1137–1138. doi: 10.1007/s00464-008-9797-z. [DOI] [PubMed] [Google Scholar]
- 5.Kane RA. Intraoperative ultrasonography: history, current state of the art, and future directions. J Ultrasound Med. 2004;23:1407–1420. doi: 10.7863/jum.2004.23.11.1407. [DOI] [PubMed] [Google Scholar]
- 6.Machi J, Oishi AJ, Furumoto NL, Oishi RH. Intraoperative ultrasound. Surg Clin North Am. 2004;84:1085–1111, vi-i. doi: 10.1016/j.suc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Kruskal JB, Kane RA. Intraoperative US of the liver: techniques and clinical applications. Radiographics. 2006;26:1067–1084. doi: 10.1148/rg.264055120. [DOI] [PubMed] [Google Scholar]
- 8.Takigawa Y, Sugawara Y, Yamamoto J, Shimada K, Yamasaki S, Kosuge T, Makuuchi M. New lesions detected by intraoperative ultrasound during liver resection for hepatocellular carcinoma. Ultrasound Med Biol. 2001;27:151–156. doi: 10.1016/s0301-5629(00)00346-x. [DOI] [PubMed] [Google Scholar]
- 9.D'Onofrio M, Vecchiato F, Faccioli N, Falconi M, Pozzi Mucelli R. Ultrasonography of the pancreas. 7. Intraoperative imaging. Abdom Imaging. 2007;32:200–206. doi: 10.1007/s00261-006-9018-y. [DOI] [PubMed] [Google Scholar]
- 10.Bartolotta TV, Taibbi A, Galia M, Runza G, Matranga D, Midiri M, Lagalla R. Characterization of hypoechoic focal hepatic lesions in patients with fatty liver: diagnostic performance and confidence of contrast-enhanced ultrasound. Eur Radiol. 2007;17:650–661. doi: 10.1007/s00330-006-0432-x. [DOI] [PubMed] [Google Scholar]
- 11.Brannigan M, Burns PN, Wilson SR. Blood flow patterns in focal liver lesions at microbubble-enhanced US. Radiographics. 2004;24:921–935. doi: 10.1148/rg.244035158. [DOI] [PubMed] [Google Scholar]
- 12.Cosgrove D, Blomley M. Liver tumors: evaluation with contrast-enhanced ultrasound. Abdom Imaging. 2004;29:446–454. doi: 10.1007/s00261-003-0126-7. [DOI] [PubMed] [Google Scholar]
- 13.Morin SH, Lim AK, Cobbold JF, Taylor-Robinson SD. Use of second generation contrast-enhanced ultrasound in the assessment of focal liver lesions. World J Gastroenterol. 2007;13:5963–5970. doi: 10.3748/wjg.v13.45.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Onofrio M, Rozzanigo U, Masinielli BM, Caffarri S, Zogno A, Malago R, Procacci C. Hypoechoic focal liver lesions: characterization with contrast enhanced ultrasonography. J Clin Ultrasound. 2005;33:164–172. doi: 10.1002/jcu.20111. [DOI] [PubMed] [Google Scholar]
- 15.Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Characterization of small focal liver lesions using real-time contrast-enhanced sonography: diagnostic performance analysis in 200 patients. J Ultrasound Med. 2006;25:349–361. doi: 10.7863/jum.2006.25.3.349. [DOI] [PubMed] [Google Scholar]
- 16.Kokudo N, Bandai Y, Imanishi H, Minagawa M, Uedera Y, Harihara Y, Makuuchi M. Management of new hepatic nodules detected by intraoperative ultrasonography during hepatic resection for hepatocellular carcinoma. Surgery. 1996;119:634–640. doi: 10.1016/s0039-6060(96)80187-5. [DOI] [PubMed] [Google Scholar]
- 17.Dai Y, Chen MH, Yin SS, Yan K, Fan ZH, Wu W, Wang YB, Yang W. Focal liver lesions: can SonoVue-enhanced ultrasound be used to differentiate malignant from benign lesions? Invest Radiol. 2007;42:596–603. doi: 10.1097/RLI.0b013e318050ab29. [DOI] [PubMed] [Google Scholar]
- 18.von Herbay A, Vogt C, Willers R, Haussinger D. Real-time imaging with the sonographic contrast agent SonoVue: differentiation between benign and malignant hepatic lesions. J Ultrasound Med. 2004;23:1557–1568. doi: 10.7863/jum.2004.23.12.1557. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Lee JM, Lee JY, Han JK, An SK, Han CJ, Lee KH, Hwang SS, Choi BI. Value of contrast-enhanced sonography for the characterization of focal hepatic lesions in patients with diffuse liver disease: receiver operating characteristic analysis. AJR Am J Roentgenol. 2005;184:1077–1084. doi: 10.2214/ajr.184.4.01841077. [DOI] [PubMed] [Google Scholar]
- 20.Bryant TH, Blomley MJ, Albrecht T, Sidhu PS, Leen EL, Basilico R, Pilcher JM, Bushby LH, Hoffmann CW, Harvey CJ, et al. Improved characterization of liver lesions with liver-phase uptake of liver-specific microbubbles: prospective multicenter study. Radiology. 2004;232:799–809. doi: 10.1148/radiol.2323030596. [DOI] [PubMed] [Google Scholar]
- 21.Nicolau C, Vilana R, Catala V, Bianchi L, Gilabert R, Garcia A, Bru C. Importance of evaluating all vascular phases on contrast-enhanced sonography in the differentiation of benign from malignant focal liver lesions. AJR Am J Roentgenol. 2006;186:158–167. doi: 10.2214/AJR.04.1009. [DOI] [PubMed] [Google Scholar]
- 22.Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420–430. doi: 10.1148/radiol.2322031401. [DOI] [PubMed] [Google Scholar]
- 23.Ding H, Wang WP, Huang BJ, Wei RX, He NA, Qi Q, Li CL. Imaging of focal liver lesions: low-mechanical-index real-time ultrasonography with SonoVue. J Ultrasound Med. 2005;24:285–297. doi: 10.7863/jum.2005.24.3.285. [DOI] [PubMed] [Google Scholar]
- 24.Wilson SR, Burns PN. An algorithm for the diagnosis of focal liver masses using microbubble contrast-enhanced pulse-inversion sonography. AJR Am J Roentgenol. 2006;186:1401–1412. doi: 10.2214/AJR.04.1920. [DOI] [PubMed] [Google Scholar]
- 25.Leen E, Ceccotti P, Kalogeropoulou C, Angerson WJ, Moug SJ, Horgan PG. Prospective multicenter trial evaluating a novel method of characterizing focal liver lesions using contrast-enhanced sonography. AJR Am J Roentgenol. 2006;186:1551–1559. doi: 10.2214/AJR.05.0138. [DOI] [PubMed] [Google Scholar]
- 26.Shukla PJ, Pandey D, Rao PP, Shrikhande SV, Thakur MH, Arya S, Ramani S, Mehta S, Mohandas KM. Impact of intra-operative ultrasonography in liver surgery. Indian J Gastroenterol. 2005;24:62–65. [PubMed] [Google Scholar]
- 27.Ellsmere J, Kane R, Grinbaum R, Edwards M, Schneider B, Jones D. Intraoperative ultrasonography during planned liver resections: why are we still performing it? Surg Endosc. 2007;21:1280–1283. doi: 10.1007/s00464-006-9192-6. [DOI] [PubMed] [Google Scholar]
- 28.Zacherl J, Scheuba C, Imhof M, Zacherl M, Längle F, Pokieser P, Wrba F, Wenzl E, Mühlbacher F, Jakesz R, et al. Current value of intraoperative sonography during surgery for hepatic neoplasms. World J Surg. 2002;26:550–554. doi: 10.1007/s00268-001-0266-2. [DOI] [PubMed] [Google Scholar]
- 29.Torzilli G, Olivari N, Moroni E, Del Fabbro D, Gambetti A, Leoni P, Montorsi M, Makuuchi M. Contrast-enhanced intraoperative ultrasonography in surgery for hepatocellular carcinoma in cirrhosis. Liver Transpl. 2004;10:S34–S38. doi: 10.1002/lt.20050. [DOI] [PubMed] [Google Scholar]
- 30.Siosteen AK, Elvin A. Intra-operative uses of contrast-enhanced ultrasound. Eur Radiol. 2004;14 Suppl 8:P87–P95. [PubMed] [Google Scholar]


