Abstract
Adult neurogenesis is a characteristic feature of the olfactory pathways of decapod crustaceans. In crayfish and clawed lobsters, adult-born neurons are the progeny of precursor cells with glial characteristics located in a neurogenic niche on the ventral surface of the brain. The daughters of these precursor cells migrate during S and G2 stages of the cell cycle along glial fibers to lateral (cluster 10) and medial (cluster 9) proliferation zones. Here, they divide (M phase) producing offspring that differentiate into olfactory interneurons. The complete lineage of cells producing neurons in these animals, therefore, is arranged along the migratory stream according to cell cycle stage. We have exploited this model to examine the influence of environmental and endogenous factors on adult neurogenesis. We find that increased levels of serotonin upregulate neuronal production, as does maintaining animals in an enriched (versus deprived) environment or augmenting their diet with omega-3 fatty acids; increased levels of nitric oxide, on the other hand, decrease the rate of neurogenesis. The features of the neurogenic niche and migratory streams, and the fact that these continue to function in vitro, provide opportunities unavailable in other organisms to explore the sequence of cellular and molecular events leading to the production of new neurons in adult brains.
Keywords: Neuronal proliferation, Neurogenic niche, Migratory stream
Introduction
Olfactory cues serve important functions in the biology of decapod crustaceans, playing central roles in feeding, mating and territorial behaviors. Correspondingly, the primary olfactory neuropil, the olfactory lobe, is one of the most prominent synaptic regions in the brains of these animals (Hanström 1925). The central olfactory pathways of decapod crustaceans, like those of most vertebrate taxa, are also characterized by the life-long addition of new neurons (Schmidt 1997; Schmidt and Harzsch 1999; Beltz and Sandeman 2003). These new neurons are the progeny of precursor cells located in specific regions of the brain that remain mitotically active throughout the lifetimes of these animals, many of which live for several decades (Wolff 1978; Cooper and Uzmann 1980). The timing and rate of proliferation in the adult decapod brain are influenced by hormonal cycles and serotonin and environmental enrichment (review: Beltz and Sandeman 2003), as they are in vertebrate brains (Kempermann 2005), indicating that these animals represent a valuable model for examining neuronal proliferation in the adult brain. The relative simplicity of the crustacean brain also provides the opportunity to examine the differentiation and integration of newborn cells within well-defined neural pathways.
The olfactory systems of decapod crustaceans share many structural and functional characteristics with those of vertebrates (Hildebrand and Shepherd 1997; Strausfeld and Hildebrand 1999; Sandeman and Mellon 2002; Ache and Young 2005; Schachtner et al. 2005). Odors are perceived in decapods by olfactory receptor neurons (ORNs) sensitive to specific odorants (Tierney et al. 1984; Derby 2000). The dendrites of the ORNs innervate specialized cuticular sensillae, known as aesthetascs, arranged along the length of the olfactory organ, the first antennae. Each aesthetasc is innervated by several hundred ORNs (Grünert and Ache 1988; Hallberg et al. 1992). An individual aesthetasc and its associated ORNs are referred to collectively as an “olfactory sensory unit” (OSU; Harrison et al. 2001). The axons of the ORNs project ipsilaterally to the brain where they terminate within the highly structured, glomerular neuropil of the olfactory lobe (OL), the primary olfactory processing area (Fig. 1) (Sandeman and Denberg 1976). The OL glomeruli are sites of synaptic contact between the ORNs, olfactory projection neurons and several classes of local interneurons. In crayfish and lobsters, the latter two classes of neurons also innervate an additional glomerular neuropil, the accessory lobe (AL), which lies adjacent to the OL (Arbas et al. 1988; Mellon and Alones 1994; Wachowiak et al. 1996; Sullivan et al. 2000; Sullivan and Beltz 2001a,b, 2005b). In contrast to the OL, which receives only primary olfactory inputs, the AL receives higher-order olfactory, visual and mechanosensory inputs (Sandeman et al. 1995; Sullivan and Beltz 2005b). Accessory lobes appear to have arisen de novo in the Eureptantia (lobsters, crayfish and crabs; Sandeman et al. 1993) and are among the most prominent neuropils in the brains of lobsters and crayfish (Blaustein et al. 1988; Sandeman et al. 1993; Sandeman and Scholz 1995). They are greatly reduced in size and complexity, however, in crabs (Sandeman et al. 1992, 1993; Sandeman and Scholtz 1995).
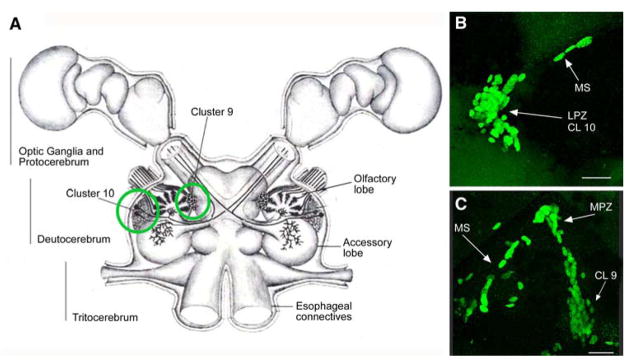
Fig. 1.
(A) Diagram of the crayfish brain including the optic ganglia. The soma clusters 9 and 10 (circled) flank two prominent neuropil regions of the deutocerebrum, the olfactory (OL) and accessory (AL) lobes. Cluster 9 contains interneurons that are local to the OL and AL. Cluster 10 contains the projection neurons that branch in either the OL or AL and have axons extending through the brain to neuropils of the lateral protocerebrum, situated in the eyestalks. (B) BrdU-labelled nuclei in the migratory stream (MS), the lateral proliferation zone (LPZ) and the adjacent cluster 10 (CL10). (C) BrdU-labelled cells in the migratory stream, the medial proliferation zone (MPZ) and cluster 9 (CL9) which is separated from the MPZ by a stream of migrating cells. Scale bar: B, C, 20 μm
The main output pathways from both the OL and AL are provided by the axons of the projection neurons, whose somata lie lateral to the lobes in a densely packed cluster, known as cluster 10 (terminology from Sandeman et al. 1992). Anatomical studies in eureptantian decapods have shown that individual cluster 10 projection neurons innervate either the OL or the AL (Wachowiak and Ache 1994; Schmidt and Ache 1996; Wachowiak et al. 1996; Sullivan et al. 2000). Cluster 10 in these animals, therefore, is comprised of at least two groups of interneurons (OL projection neurons, AL projection neurons) with distinct functional identities (Sullivan and Beltz 2005b). The axons of the OL projection neurons join with those of the AL projection neurons upon leaving the lobes to form a large tract, known as the olfactory globular tract (OGT), which bifurcates in the centre of the brain before projecting bilaterally to higher-order regions of the brain, known collectively as the lateral protocerebrum (Hanström 1925; Sullivan and Beltz 2004). Most cluster 10 projection neurons express the neuropeptide crustacean-SIFamide (Yasuda et al. 2004; Sullivan and Beltz 2005b,c; Yasuda-Kamatani and Yasuda 2006; Sullivan et al. 2007).
In addition to the projection neurons, the OL and AL are also innervated by several classes of local interneurons: OL- and AL-specific interneurons and interneurons innervating both regions (Schachtner et al. 2005). The somata of these interneurons form a cluster located medial to the lobes, known as cluster 9 (terminology from Sandeman et al. 1992). Neurons in this heterogeneous soma cluster express a wide array of peptide (allatostatin, crustacean-SIFamide, enkephalin, FMRFamide, orcokinin, proctolin, tachykinin), amino acid (GABA), monoamine (histamine, serotonin, dopamine), and gaseous (nitric oxide) neurotransmitters (Langworthy et al. 1997; Schmidt and Ache 1997; Schachtner et al. 2005; Yasuda-Kamatani and Yasuda 2006).
Life-long proliferation in the olfactory system
Most of the neurons in the central nervous system of adult decapods are born during embryonic development and are the progeny of large precursor cells, known as neuroblasts (for review see Harzsch 2003). Neuroblasts arise during early embryonic development and divide asymmetrically generating specific lineages of neurons before degenerating during late embryonic or early postembryonic development. Proliferation in most regions of the decapod brain ceases, therefore, in the period around hatching. The exception to this, however, is the central olfactory pathway where mitotic activity continues through hatching and thereafter occurs throughout the lifetimes of these animals (Schmidt 1997; Harzsch et al. 1999; Beltz and Sandeman 2003). Adult neurogenesis also occurs in the visual pathway (Sullivan and Beltz 2005c), though this has been studied in much less detail.
Application of cell cycle markers to the brains of adult eureptantian decapods (lobsters, crayfish and crabs) indicates that proliferation occurs continuously within both clusters 10 and 9, the soma clusters of the olfactory interneurons (Fig. 1) (Schmidt and Harzsch 1999; Sullivan and Beltz 2005a). In crabs, proliferation also occurs within the soma cluster of local interneurons innervating the lateral protocerebrum (Schmidt 1997; Schmidt and Harzsch 1999; Hansen and Schmidt 2001; Sullivan and Beltz 2005a), the target neuropil of the cluster 10 projection neurons. Proliferation within these cell clusters occurs within restricted regions, known as proliferation zones: the lateral proliferation zone (LPZ; cluster 10), the medial proliferation zone (MPZ; cluster 9), and the lateral protocerebral proliferation zone (PPZ; lateral protocerebrum). Pulse-chase BrdU experiments indicate that newborn cells are translocated away from the proliferation zones over time, that they become dispersed amongst the other cells in the clusters, and that they survive for at least a year (Harzsch et al. 1999; Schmidt 2001; Sullivan and Beltz 2005a,c). It remains unclear whether this translocation occurs passively or involves active migration to specific regions within the clusters (Schmidt 2001). Quantitative analyses of cluster 10 indicate that the number of neuronal somata within the cluster increases throughout the animals’ lifetimes (Schmidt 1997; Sandeman et al. 1998).
As in most vertebrate taxa, proliferation in the central olfactory pathways of adult decapod crustaceans occurs in parallel with the continuous proliferation and turnover of ORNs in the olfactory organ (crustaceans: Sandeman and Sandeman, 1996; Steullet et al. 2000; Harrison et al. 2001; Derby et al. 2003; vertebrates: Graziadei and Monti-Graziadei 1978; Farbman 1992; Byrd and Brunjes 2001; Beites et al. 2005). Most decapod crustaceans, such as crayfish and lobsters, have indeterminate growth and continue to moult and grow throughout their lives (Hartnoll 1982). The number of ORNs in these animals also increases throughout their lifetimes (Harrison et al. 2001; Derby et al. 2003). The addition of new ORNs to the olfactory organ occurs primarily through the formation of new OSUs (aesthetascs and associated ORNs) rather than through the addition of neurons to existing OSUs (Derby et al. 2003). The generation of new OSUs occurs at the proximal end of the olfactory organ within a restricted proliferation zone (Harrison et al. 2001). The subsequent differentiation and maturation of newborn ORNs occurs over several weeks and is associated with elevated levels of taurine within the cytoplasm (Steullet et al. 2000).
In adult, but not juvenile, crayfish and lobsters the proximal addition of new OSUs occurs in parallel with the loss of the oldest, distal-most OSUs, resulting in a continuous turnover and replenishment of ORNs (Sandeman and Sandeman 1996; Steullet et al. 2000). In between the proximal (proliferation) and distal (senescence) regions of the olfactory organ, ORNs mature and become odor sensitive (Steullet et al. 2000). The birth, differentiation and death of ORNs, therefore, occur in a spatiotemporal wave along the length of the olfactory organ. There is a continual increase in the numbers of OSUs (and hence ORNs) as animals grow, however, as the rate of addition is always greater than the rate of loss (Harrison et al. 2001; Derby et al. 2003).
As the life-long addition of ORNs occurs in parallel with the continuous addition of olfactory interneurons in the brain, it has been proposed that these two processes are related functionally, with new olfactory interneurons being added to the central olfactory pathway to accommodate the ingrowing axons of new ORNs (Schmidt 1997; Sandeman et al. 1998; Steullet et al. 2000; see also reviews of vertebrate studies by Lledo and Saghatelyan 2005; Lindsey and Tropepe 2006). This hypothesis, however, has rarely been tested. To address this question, we examined proliferation in the olfactory system of immature and mature spider crabs, Libinia emarginata (Sullivan and Beltz 2005a). Unlike most decapod species, L. emarginata exhibits determinate, rather than indeterminate, growth and has a terminal, maturational moult after which animals become anecdysic (i.e. stop moulting: Carlisle 1957; Hartnoll 1963; Hinsch 1972).
As new cuticular sensillae, such as aesthetascs, can only be added at moulting, the addition of ORNs to the decapod olfactory organ is also dependent on moulting (Sullivan and Beltz 2005a). If, as proposed, the continuous addition of olfactory interneurons is a mechanism by which the central olfactory pathway accommodates the addition of ORNs, it would follow that the proliferation of olfactory interneurons in L. emarginata would not continue beyond the terminal moult. A comparison of neuronal proliferation in immature and mature L. emarginata, therefore, enables an examination of the interdependence of central and peripheral neurogenesis in the olfactory pathway. These studies demonstrated that the continuous addition of ORNs in L. emarginata ceases at the terminal, maturational moult but that the proliferation of olfactory interneurons continues in mature animals (Sullivan and Beltz 2005a). Pulse-chase BrdU experiments in mature, anecdysic L. emarginata show that after several months newborn cells in cluster 10 express the neuropeptide crustacean-SIFamide and have arbors innervating the OL, indicating that these cells have differentiated into functioning neurons. Together, these results demonstrate that continuous proliferation of ORNs is not a requirement for life-long neurogenesis amongst interneurons in the central olfactory pathway.
Regulation of life-long neurogenesis in the central olfactory pathway
Living conditions
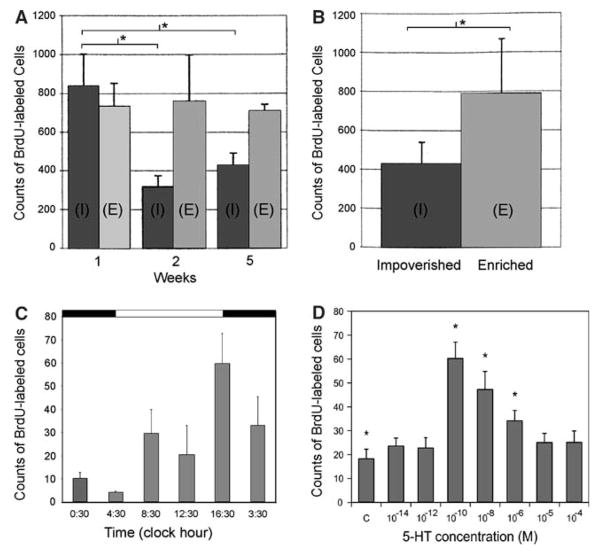
A number of environmental and endogenous factors influence olfactory neurogenesis in adult decapods. As in mammals (Kempermann and Gage, 1999), the rate of neuronal proliferation in these animals is highly sensitive to environmental enrichment (Sandeman and Sandeman, 2000; Van der Meeren et al. 2007). When crayfish are housed for even short periods (1–2 weeks) in large spaces with conspecifics and stimulating/naturalistic surroundings (enriched environment), the level of proliferation in both the LPZ and MPZ is significantly higher than in crayfish isolated in small, barren spaces (impoverished environment; Fig. 2A). Environmental enrichment also enhances the survival of newborn cells (Fig. 2B) (Sandeman and Sandeman 2000).
Fig. 2.
(A) Counts of BrdU-labelled cells in clusters 9 and 10 on both sides of the crayfish brain (Cherax destructor) in animals exposed to impoverished (I) and enriched (E) conditions over a period of 5 weeks. A decline in the numbers of cells in animals in the impoverished conditions is recorded after 2 weeks. (B) Counts of surviving BrdU-labelled cells in clusters 9 and 10 on both sides of the brain 2 weeks after immersion in BrdU for 24 h, and 4 weeks of subjection to the different environmental conditions. Asterisks in (A) and (B) indicate significant differences. (C) Counts of BrdU-labelled cells in cluster 10 of the lobster brain (Homarus americanus) are correlated with the time of day, with proliferation levels being highest at dusk and lowest at dawn. D. Levels of serotonin (5-HT) influence neurogenesis in the LPZ of the lobster brain in vitro. Dissected brains from 6 to 7th stage juvenile lobsters were incubated in serotonin (10−14–10−4 M; n = 8 brains/condition) dissolved in an enriched lobster saline solution containing 0.2 mg/ml BrdU at 13°C for 4 h. In comparison to control brains (C; no serotonin added), serotonin at 10−10–10−6 M significantly increased BrdU incorporation in the LPZ (asterisks; ANOVA followed by a Tukey’s post-hoc)
Several studies have attempted to separate the many variables present in environmental enrichment studies (e.g. levels and types of sensory inputs, locomotory levels) in order to understand the basis of these effects. Effects of intraspecific interactions on neurogenesis have been investigated in experiments examining the formation of dominant:subordinate relationships, which are dependent upon olfactory cues (Zulandt Schneider et al. 1999; Breithaupt and Eger 2002). These studies have demonstrated effects of social status on the levels of proliferation (Beltz and Sandeman 2003) and survival (Song et al. 2007) of newborn cells in the central olfactory pathways of crayfish. In contrast, studies in the clawed lobster, Homarus americanus, have not found effects of dominant: subordinate status on olfactory neurogenesis (Beltz and Sandeman 2003), although the process of establishing the social relationship appears to influence the rates of both neuronal birth and survival (Kim 2004). Differences among studies in experimental conditions and protocols, however, have made direct comparisons of results difficult and common conclusions cannot be drawn. The definitive study of this topic, therefore, has yet to be done.
Animal size and age
Another important element in environmental enrichment studies is the effect of environment on growth, as animal size is a primary determinant of the basal levels of neurogenesis in the LPZ and MPZ, with proliferation levels being lower in larger animals than in smaller animals (Sandeman and Sandeman 1998; Harzsch et al. 1999; Hansen and Schmidt 2004). As growth in crustaceans is influenced by numerous factors, including the degree of environmental enrichment, there is no clear correlation between size and age in these animals; two animals of the same age can differ greatly in size. Consequently, it has been difficult to determine the extent to which the observed decreases in neurogenesis over time are linked to size, age and/or growth rate. Consistent with findings that confined spaces constrain growth in crustaceans (Wahle and Fogarty 2006), small spaces also reduce the levels of neurogenesis among the olfactory interneurons (Beltz and Sandeman 2003; Van der Meeren et al. 2007). While a gradual decrease in the basal level of neurogenesis is associated with increased size in crustaceans, this is a continuum (Sandeman et al. 1998) and there is no evidence that the mechanism generating new neurons differs fundamentally in small/young and large/old animals. It remains unknown whether the observed decreases in proliferation levels with size/age result from decreases in the numbers of precursor cells in the brain and/or changes in their proliferative activity. Interestingly, while the rate of proliferation in the LPZ of the portunid crab Carcinus maenas decreases with animal size, the rate in the PPZ remains constant (Hansen and Schmidt 2004).
Sensory inputs from the olfactory organ also appear to play an important role in the regulation of neuronal proliferation and survival in the central olfactory pathway. Experimental studies indicate that unilateral ablation of the olfactory organ causes a reduction in the rate of proliferation and the survival of newborn cells in the LPZ (Hansen and Schmidt 2001) and a decrease in the numbers of local (cluster 9) and projection (cluster 10) neurons (Sandeman et al. 1998). This decrease in neuronal number is accompanied by an increase in the numbers of apoptotic cells (Sandeman et al. 1998), though it is not known whether these apoptosing cells represent newborn or mature neurons. As these studies have not examined the effects of olfactory organ ablation on the processes of the ORNs within the OL, the extent to which the observed effects may be attributable to an absence of physiological activity in the ORNs and/or to their degeneration and loss remains unclear.
Sensitivity to light
Circadian and circa annual rhythms in the rates of olfactory neuronal proliferation have been demonstrated in crustacean species. The day:night cycle is an important regulator of the rate of BrdU incorporation in the clawed lobster, H. americanus; proliferation levels in the LPZ are highest during the hours around dusk, the period of the day during which these nocturnal animals are most active (Fig. 2C) (Goergen et al. 2002). These findings raise the possibility that light-controlled circadian rhythms represent a primary regulator of neuronal proliferation, and that endogenous factors that influence neurogenesis (e.g. serotonin) are secondary events in this pathway. Seasonal changes in neurogenesis have also been demonstrated in the crab C. maenas, with proliferation rates peaking in spring and late summer in the LPZ and in early summer in the PPZ (Hansen and Schmidt 2004). The rhythmic moult cycle of crustaceans also influences the levels of proliferation of both ORNs (Harrison et al. 2001) and olfactory interneurons (Beltz and Sandeman 2003).
Diet
Diet represents another important aspect of the regulatory story. Experiments in H. americanus demonstrate that even short-term dietary augmentation of omega-3 relative to omega-6 fatty acids results in significant increases in the rate of neuronal proliferation in the LPZ (Beltz et al. 2007). The ratio of omega-3 to omega-6 fatty acids has been proposed to alter neurogenesis by influencing membrane fluidity (Yehuda et al. 1998) and/or the structure of membrane proteins (Bourre et al. 1991). The omega-3:omega-6 ratio also modulates cytokine and neurotrophin levels (Harbige 2003), which in turn regulate neuronal proliferation and cell fate (Beck et al. 2005).
Serotonin and nitric oxide
It is clear that many factors are capable of altering the rate of neuronal production in the crustacean brain; indeed, the situation is much like that in mammals, where the comment has been made that “…some critical minds have longed to see the one stimulus that does not affect adult neurogenesis” (Kempermann et al. 2004). A primary goal in our lab is to move away from categorising the various phenomena that influence neurogenesis, and instead to decipher the pathways that translate these exogenous and endogenous signals into changes in the rate of neurogenesis. Towards this end, we have examined serotonin and nitric oxide as possible endogenous modulators of neurogenesis. Various in vitro and in vivo studies have shown that increases in serotonin levels result in an upregulation of neurogenesis in both the MPZ and LPZ of juvenile clawed lobsters (Benton and Beltz 2001a; Beltz et al. 2001) and that decreases inhibit the branching of newborn projection neurons (Sullivan et al. 2000). Furthermore, brain serotonin levels are under circadian control (Wildt et al. 2004). Current studies are aimed at determining whether serotonergic pathways form an integral part of the mechanism underlying circadian regulation of neurogenesis, or whether this pathway functions in parallel with photoperiodic controls. These studies may ultimately reveal mechanisms by which other exogenous factors are translated into changes in neurogenesis. In this regard, it is important to note that we believe that serotonin may influence olfactory neuronal precursors both via release from fine fibers of the serotonergic dorsal giant neuron (DGN; Beltz et al. 2001) and via a blood-born pathway. Recent in vitro studies in the clawed lobster demonstrate that neurogenesis in the LPZ is most sensitive to serotonin at very low (10−10M) concentrations (Fig. 2D). Higher levels of serotonin are less effective in upregulating neurogenesis, with 10−4–10−5 M levels causing no significant increase in the numbers of BrdU-labelled cell profiles compared to control brains incubated in saline without serotonin. This high sensitivity suggests that hormonal regulation is a primary mechanism by which serotonin alters neurogenesis in these animals.
Nitric oxide (NO) has also been implicated in pathways controlling olfactory neurogenesis in crustaceans (Benton and Beltz 2001b, 2005; Benton et al. 2003; Beltz and Sandeman 2003). Immunolabelling for nitric oxide synthase (NOS), the enzyme responsible for the synthesis of NO, occurs in a number of regions and cells within the olfactory pathway that also express serotonin immunoreactivity, including the DGN (Beltz and Sandeman 2003) whose fine fibers innervate the LPZ (Beltz et al. 2001). Experimental manipulations of NO levels using NO donors and NOS inhibitors demonstrate effects of this gaseous neurotransmitter on olfactory neurogenesis, with increased levels of NO resulting in decreases in the levels of neuronal proliferation (Benton et al. 2003). Furthermore, experiments in the clawed lobster, H. americanus, suggest the decreases in neuronal proliferation observed following ablation of the olfactory organ (Sandeman et al. 1998; Hansen and Schmidt 2001) may be mediated, at least in part, by nitric oxide. The distribution of NOS immunoreactivity in the central olfactory pathway of H. americanus exhibits a consistent sequence of changes during the days following olfactory organ ablation (Benton and Beltz 2007b). How the serotonergic and nitrergic pathways may be interrelated in the crustacean brain is not known. However, the fact that NO and serotonin coexist in the DGN and that these two compounds have opposing effects on neuronal proliferation, suggest intriguing possibilities for the control of neurogenesis that require further investigation.
Precursor cells maintaining adult neurogenesis in the central olfactory pathway
The in vivo identity and location of the precursor cells responsible for maintaining adult neurogenesis in decapods has, until recently, remained elusive. There is no evidence that embryonic neuroblasts, which are responsible for the production of the initial complement of neurons in the embryonic brain, persist beyond the earliest stages of postembryonic development (Harzsch et al. 1999; Sandeman and Sandeman 2003: however, see P. argus below). The problem of maintaining neurogenesis in adult brains over several decades appears, therefore, to require the establishment of a different proliferative system, distinct from the neuroblasts that are responsible for the generation of the CNS during embryogenesis. The first evidence providing insights into the nature of this system arose from recent experiments examining olfactory neurogenesis in juvenile and sexually mature crayfish, Procambarus clarkii (Sullivan et al. 2007).
Anatomy of the proliferative system maintaining adult neurogenesis
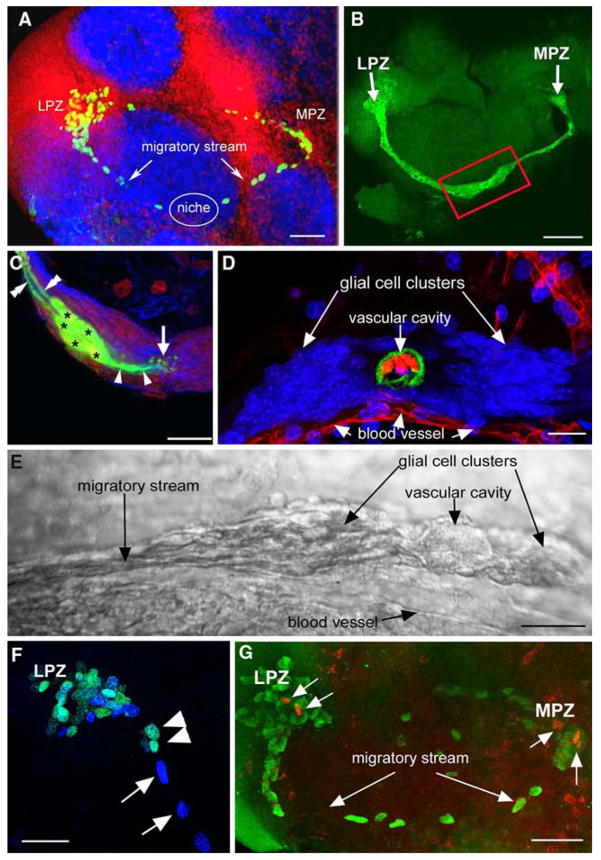
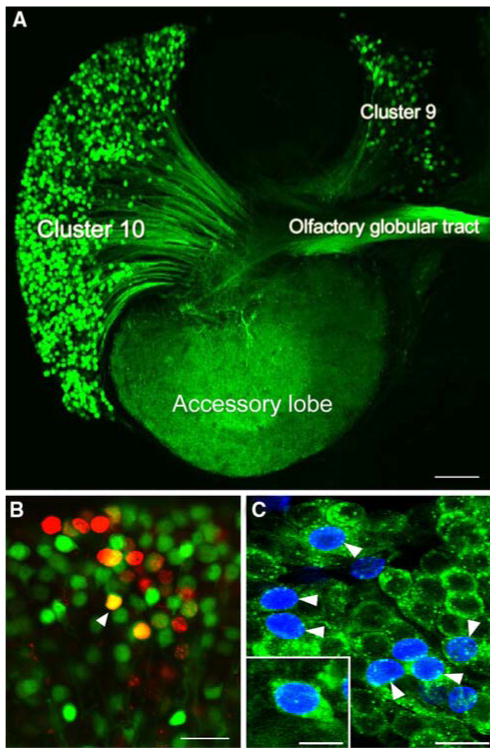
Studies of the distribution of glia within the brain of adult P. clarkii show that both the LPZ and MPZ are contacted by a specialized population of glial cells whose somata form a cluster on the ventral surface of the brain (Fig. 3A, B) (Sullivan et al. 2005, 2007). These cells differ from other brain glia in the close proximity of their somata and the fasciculation of their processes into tracts (Linser et al. 1997; Sullivan and Beltz 2005c; Allodi et al. 2006) and occur in both juvenile and adult crayfish (Sullivan et al. 2005, 2007). Lateral and medial subpopulations within the glial soma cluster (GSC) are grouped around a central, circular region that does not label with glial markers (Fig. 3D). Intracellular dye fills of individual glial cells show them to be bipolar, with short processes projecting to the central, unlabelled region of the cluster and longer processes that fasciculate to form the tracts projecting to the LPZ and MPZ (Fig. 3C). Glial cells with somata in the lateral region of the cluster project to the LPZ while glia in the medial region project to the MPZ, suggesting that the two halves of the cluster may be able to function independently. Labelling of the crayfish brain vasculature by injecting dextran dye into the dorsal artery indicate that the central, circular region of the cluster, to which the short processes of the glia project and which is not labelled by glial markers, is confluent with the brain vasculature (Fig. 3D). Consequently, we have named this region the vascular cavity.
Fig. 3.
The proliferative system maintaining adult neurogenesis in the central olfactory pathway of the crayfish Procambarus clarkii. (A) Left side of the brain of P. clarkii labelled immunocytochemically for BrdU (green). Labelled cells are found in the lateral proliferation zone (LPZ) contiguous with cluster 10 and in the medial proliferation zone (MPZ) near cluster 9. The two zones are linked by a chain of labelled cells in a migratory stream that originates in the oval region labelled “niche”. Labelling for Drosophila synapsin (blue) and propidium iodide (red) is also shown. (B) Both the LPZ and MPZ are contacted by the processes of a specialized population of glial cells immunoreactive to glutamine synthetase (green). The somata of these cells form a cluster, the niche (red box), on the ventral surface of the brain. (C) Glial cells (green) in the niche labelled by intracellular injection of Lucifer yellow, have short processes projecting to the vascular cavity and longer fibers that fasciculate together to form the tracts projecting to the LPZ and MPZ. (blue: glutamine synthetase; red: propidium iodide). (D) The vascular cavity in the centre of the glial soma cluster in a brain in which the brain vascular system was filled by injecting a dextran dye solution into the cerebral artery. The cavity, outlined in green by its reactivity to an antibody to Elav, contains the dextran dye (red) which is also contained within a larger blood vessel that runs along beneath the niche. Propidium iodide (blue) labelling of the glial cell nuclei is also shown. (E)Differential interference contrast image of a living niche dissected from the ventral surface of the brain. The glial clusters, vascular cavity, migratory stream and blood vessel shown in the labelled preparation in (D) are all clearly distinguishable in the living system. (F) BrdU (cyan) and IodU (blue) labelling in the lateral migratory stream and LPZ in a double nucleoside analogue-labelling experiment. Animals were exposed to BrdU for 6 h and then removed and maintained in fresh pond water for 6 days after which they were immersed in IodU for 6 h, killed and processed with antibodies to BrdU and IodU. The nuclei in the migratory stream after 6 days label only with the IodU indicating that the initially present BrdU labelled nuclei had moved out to the LPZ where they predictably label for both BrdU and IodU. (G) The proliferation zones and the stream labelled with antibodies raised against BrdU (green) and the M-phase marker, phospho-histone H3 (ser10) (red). Histone labels cells exclusively in the lateral and medial proliferation zones. Scale bars: A, 100 μm; B, 75 μm; C, 20 μm; D, 20 μm; E, 25 μm; F, 40 μm; G, 50 μm
Proliferation within the glial soma cluster
Examination of the GSC using several cell cycle markers indicates that this region represents an additional proliferative site in the adult crayfish brain (Sullivan et al. 2007). Long periods of exposure to BrdU (10–14 days) reliably label 1–2 cells in both the lateral and medial subpopulations of the cluster. These cells also label with an M-phase marker (phospho-histone H3, thr11), indicating that they undergo mitosis. The length of time required to label cells within the cluster with BrdU suggests that they are relatively quiescent, as are neuronal precursor cells in adult vertebrates, which may rest in the G1 phase of the cell cycle for several days between cell divisions (Morshead et al. 1994; Maslov et al. 2004). Indeed, a G1 phase-specific marker (MCM2-7; Cvetic and Walter 2006) reliably labels cells in each subpopulation of the glial soma cluster (Sullivan et al. 2005, 2007). Together, these results demonstrate that the GSC contains a small number of mitotically active cells and that these cells exhibit in vivo properties of precursor cells.
Migration from the glial soma cluster to the olfactory proliferation zones
In addition to labelling a small number of cells within the GSC, exposure to BrdU also labels the nuclei of cells arranged along the lengths of the glial tracts projecting to the LPZ and MPZ. Pulse-chase BrdU and double nucleoside analogue-labelling experiments show that this migration is directed along the tracts away from the GSC towards both the LPZ and the MPZ (Fig. 3F). Accordingly, these glial tracts have been named the lateral and medial migratory streams (Sullivan et al. 2007). Double nucleoside analogue-labelling also demonstrates that it takes 2–3 days for cells to reach the proliferation zones (Benton et al. unpublished results).
Given that exposing crayfish to BrdU for even short periods (12 h) usually results in the labelling of cells along the entire lengths of the migratory streams, we have to suppose that a constant supply of new daughter cells (1–3 per day), are being produced in the GSC and start their journey to either the LPZ or MPZ. The long exposure to BrdU that is required to reliably label S-phase cells in the GSC suggests that S-phase is relatively short compared to the length of the cell cycle. These data also suggest that a sizable pool of progenitor cells (i.e., >4 per niche) must be available in the GSC. In some preparations, particularly those that received very short BrdU labelling times, or in older animals, we find that the migratory streams contain very few or no labelled cells. The absence of cells in the migratory streams in these preparations could be explained by a slower cell cycle time in the GSC such that the rate of generation of daughter cells falls below the rate of migration to the proliferation zones, a feature, it would appear, of larger/older animals.
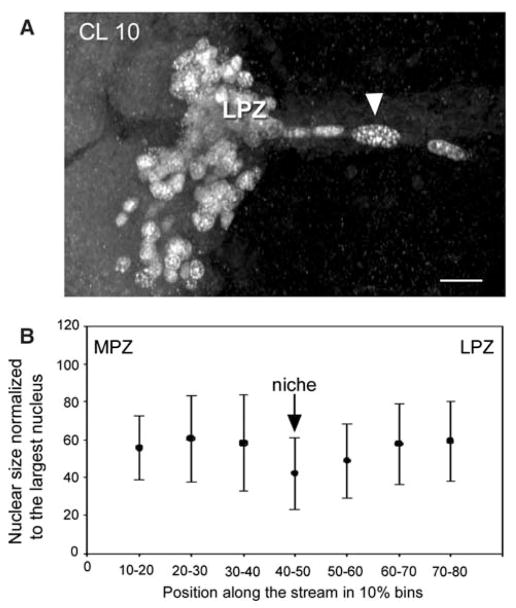
The migratory stream of P. clarkii is also characterised in many preparations by the presence, close to the MPZ and LPZ, of three or four BrdU-labelled nuclei of very different sizes; the nucleus furthest from the proliferation zones being large and rounded while the two or three nuclei closest to the zones are smaller and elongated (Fig. 4A). Size differences of BrdU-labelled nuclei in the migratory streams and proliferation zones are a consistent characteristic of this system in P. clarkii. Similar large nuclei have been reported to occur in a comparable location in the brains of adult spiny lobsters, Panulirus argus, and have been interpreted as “adult neuroblasts” serving as neuronal stem cells providing the progenitor cells for the LPZ and MPZ (Schmidt 2007a, b). These large nuclei in P. clarkii do not always occur in the same place in different individuals; however, when present they are always located within the migratory stream, leading us to a different interpretation of their identity than has been suggested for P. argus (see below, Precursor cells maintaining adult neurogenesis in the spiny lobster). In addition, measurement of the size (area) and location of over 100 BrdU-labelled nuclei in the lateral migratory streams of seven P. clarkii indicate a tendency of nuclear size to increase toward the LPZ and MPZ (Fig. 4B).
Fig. 4.
(A) BrdU-labelled nuclei in the lateral migratory stream close to the LPZ. One large nucleus (arrow head) is typically separated from the LPZ by two or three smaller nuclei. (B) Measures of nuclear size in the migratory streams reveal their tendency to increase as they approach the proliferation zones. The abscissa shows the distance along the migratory stream from the MPZ to the LPZ divided into 10% bins, with 0% being at the MPZ and 100% at the LPZ. The graph shows that nuclei in the region of the niche tend to be smaller than those closer to the MPZ and LPZ. At the ends of the streams, close to the proliferation zones, the nuclei tend to decrease in size again as shown in A. Scale bar: A, 20 μm
Application of M-phase markers (phospho-histone H3 [ser 10] or [thr 11]) results in the labelling of cells in the GSC, the LPZ and the MPZ (Fig. 3G) (Sullivan et al. 2007). No M-phase labelling, however, has thus far been observed of cells in the migratory streams (Fig. 3G), neither in our preparations nor in the published images of others who have worked on neurogenesis in P. clarkii (Song et al. 2007). A model that would fit these observations (summarised in Fig. 5) is that cells migrate from the GSC to the proliferation zones during the interphase stages of S and G2. It is during the S phase that cells replicate their DNA and often both the nucleus and cell soma increase in size during this time, maintaining this size increase until mitosis occurs (Alberts et al. 1998). Given the measurements of nuclear size in our preparations and the absence of phospho-histone H3 labelling of nuclei along the migratory streams, we conclude that neuronal precursor cells residing in the GSC divide (Sullivan et al. 2007) and then remain in G1 until they enter S phase (hence the BrdU label), perhaps propelled by a blood-borne chemical signal; they then migrate along the stream during the transition from S to G2 with a concomitant increase in nuclear size. By the time these cells reach the LPZ and MPZ they have increased in size and are about to enter M phase. Predictably, phospho-histone H3 labelling occurs in both the LPZ and MPZ.
Fig. 5.
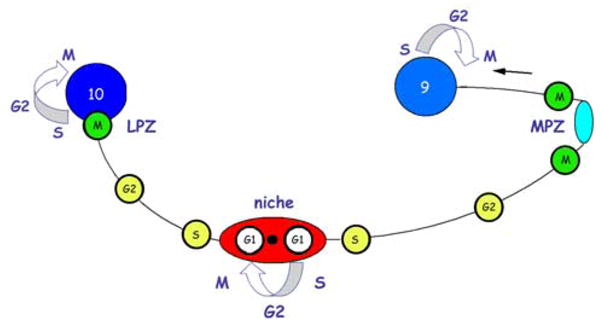
A model summarizing our current view of events leading to the production of new olfactory interneurons in adult crayfish, clawed lobsters and crabs. Relatively quiescent precursor cells exhibiting glial characteristics reside within a neurogenic niche where they divide asymmetrically, resting in G1 phase of the cell cycle between divisions, to produce one self-renewed precursor cell and one daughter cell. Daughter cells begin migrating to the proliferation zones, progressing from S phase of the cell cycle to the G2 phase as they migrate. Close to the proliferation zones these cells undergo mitosis to become third generation progenitors; one or more divisions of these cells will generate immature neurons in clusters 9 or 10. Many of these cells subsequently differentiate into olfactory interneurons and become incorporated into the nervous system as functional units
Size differences are also a feature of BrdU-labelled nuclei in the LPZ and MPZ (Fig. 1B, C; Fig 4A). Our interpretation is that in crayfish and clawed lobsters, the large BrdU-labelled cells near the proliferation zones are progenitor cells that will undergo an additional, final cell cycle to produce immature neurons, many of which will become incorporated as functional elements of the olfactory pathway (see Differentiation of newborn cells, below).
Comparison between the neurogenic niche of P. clarkii and those of vertebrates
Our results suggest that cells within the glial soma cluster are the precursor cells maintaining adult neurogenesis in P. clarkii and that this cluster, therefore, represents a neurogenic niche. Furthermore, they suggest that the precursor cells themselves exhibit features of glial cells. The progeny of these cells appear to migrate along glial tracts (the lateral and medial migratory streams) to the LPZ and MPZ, where proliferation occurs. This arrangement suggests that the migrating cells may represent an amplification stage analogous to the transit-amplifying cells (type-C cells) of the neurogenic niches of adult mice (Doetsch 2003b). Elements of this pathway have also been observed in a portunid crab (C. maenas; Beltz et al. 2005b), the Australian crayfish (C. destructor; Fig. 6A) and a clawed lobster (H. americanus; Fig. 6B), and we therefore believe that this mechanism for generating neurons is broadly applicable among closely related species. Further, although elements of the migratory stream have been displayed in images from other labs (Hansen and Schmidt 2004; Song et al. 2007), the significance of these pathways was not understood until the discovery of the neurogenic niche and experiments illustrating the migration of the glial daughters to the medial and lateral proliferation zones (Sullivan et al. 2005, 2007).
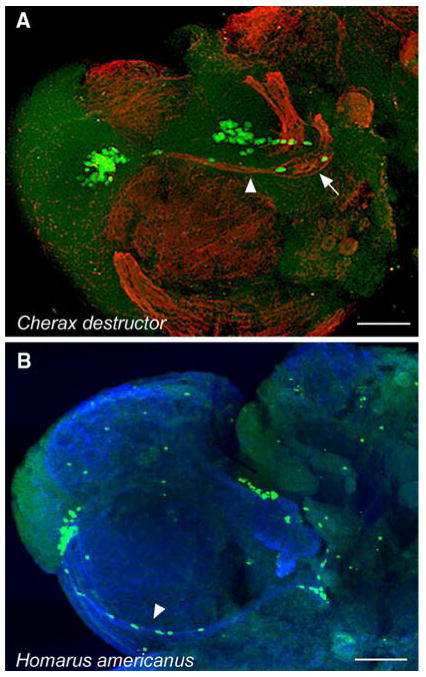
Fig. 6.
BrdU-labelled cells in the proliferation zones and migratory streams of Cherax destructor (A) and Homarus americanus (B). Arrowheads in A and B mark the migratory stream labelled with β-tubulin (fibers) and BrdU (cells). The niche can be seen in A (arrow) but is hidden in the fold between the accessory lobe and the antenna 2 neuropil in B. The features of this system in C. destructor and H. americanus are virtually identical to those we have described in detail for P. clarkii. Scale bar: A, B, 100 μm
The niche of P. clarkii possesses remarkable structural and functional parallels with the neurogenic niches of adult vertebrates. These include: glial cells functioning as both precursor and support cells (Garcia-Verdugo et al. 2002; Song et al. 2002; Doetsch 2003 a,b; Garcia et al. 2004; Merkle et al. 2004; Seri et al. 2004; Ma et al. 2005), directed migration (Lois et al. 1996; Bolteus and Bordey 2004), close association with the brain vasculature (Palmer et al. 2000; Palmer 2002; Shen et al. 2004), and specialised basal laminae (Mercier et al. 2002; Campos 2005). The cellular machinery maintaining adult neurogenesis appears, therefore, to be shared by widely disparate taxa. These extensive structural and functional parallels suggest a common strategy across phyla for the generation of new neurons in adult brains.
Precursor cells maintaining adult neurogenesis in the spiny lobster, Panulirus argus
Examinations of the brains of adult spiny lobsters, P. argus, have not provided evidence of glial soma clusters or migratory streams in the olfactory pathways of these animals. Multiple BrdU injections over 2 days, however, result in the labelling of single (or very few) large nuclei adjacent to the LPZ and MPZ (Schmidt 2007a, b). These cells have been putatively identified as “adult neuroblasts” on the basis of their large size relative to cells in the LPZ and MPZ (Schmidt 2007a, b). Each adult neuroblast is surrounded by a cluster of small cells, which also form a tube-like structure connecting the neuroblasts with the proliferation zones (Schmidt 2007a, b). It has been proposed that these adult neuroblasts undergo rapid asymmetric divisions producing series of daughter cells which translocate to the proliferation zones where they divide symmetrically over several days to produce two immature neurons (Schmidt 2007a, b). The cellular basis of adult neurogenesis in P. argus appears, therefore, to be similar to that of neurogenesis during embryonic development. Whether functional parallels or homologies exist between the neurogenic system of P. argus and those of other decapods species examined (for example, between the putative “adult neuroblasts” of P. argus and the putative “G2 cells” of P. clarkii) is a subject for further investigation.
Differentiation of newborn cells in the central olfactory pathway
While proliferation and the regulation of mitotic activity in the LPZ and MPZ have been examined in detail (reviewed in Beltz and Sandeman 2003), it is only relatively recently that studies have begun providing insights into the differentiation of adult-born cells. Research into the differentiation of newborn cells in these proliferation zones has been hampered by the lack of specific markers for crustacean neurons (Schmidt 2001; Sullivan and Beltz 2005c). By combining pulse-chase BrdU experiments with immunolabelling for specific neurotransmitters and tract tracing, however, it has been possible to examine the fate and final phenotypes of these adult-born cells (Fig. 7). Neuronal differentiation in the central olfactory pathway has been most extensively examined in crayfish, C. destructor and P. clarkii, and the spiny lobster, P. argus.
Fig. 7.
(A) The left side of a brain of P. clarkii in which dextran was applied to the accessory lobe. The dextran (green) enters neurons that have their terminals in the accessory lobe and labels the corresponding cell bodies and axons. From this it is clear that both projection neurons in cluster 10, and local interneurons in cluster 9, have their terminals in the accessory lobes and the axons from the projection neurons lie in the olfactory globular tract. (B) Cluster 10 cell bodies from an animal that was exposed to BrdU for 12 days and then maintained in fresh pond water for 4 months. At this stage the animal was killed and dextran fluorescein 3000 MW was applied to the accessory lobe (Sullivan and Beltz 2005c). Cells labelled red indicate that they passed through the cell cycle in the presence of BrdU. Cells labelled green indicate that they have terminals in the accessory lobe but did not pass through a cell cycle in the presence of BrdU. Double-labelled cells (orange) are cells that passed through a cell cycle in the presence of BrdU and have differentiated into neurons with their terminals in the accessory lobe. (C) Cluster 10 cell bodies with BrdU (blue) and crustacean-SIFamide (green) label six months after being exposed to BrdU. Double-labelled cells, green and blue (arrowheads). Crustacean-SIFamide immunoreactivity is known to be expressed in olfactory interneurons in P. clarkii (Yasuda-Kamatani and Yasuda, 2006) and the presence of double labelling indicates that these cells were born in the adult animal and have differentiated into olfactory interneurons. Scale bars: A, 10 μm; B, C, 20 μm; C insert, 10 μm
Differentiation of newborn cells in the LPZ (cluster 10): projection neurons
Pulse-chase BrdU experiments in adult crayfish indicate that large numbers of newborn cells in cluster 10 differentiate into neurons expressing crustacean-SIFamide (Fig. 7C) (Sullivan and Beltz 2005c; Sullivan et al. 2007). Similarly, as described above, many newborn cells in cluster 10 of the spider crab, L. emarginata, also differentiate into crustacean-SIFamide expressing neurons (Sullivan and Beltz 2005a). In both groups of animals, the differentiation of these newborn cells appears to occur over 4–6 months (Sullivan and Beltz 2005a, c; Sullivan et al. 2007). No evidence has been obtained using glial markers (glutamine synthetase, glial fibrillary acidic protein) indicating that newborn cells in cluster 10 differentiate into glia (Sullivan and Beltz 2005a). Together, these results suggest that proliferation in these regions solely represents neurogenesis.
In order to more closely examine the final phenotypes of newborn neurons in cluster 10, pulse-chase BrdU experiments in C. destructor were combined with the application of tract-tracing dextran dyes to the OL and AL (Fig. 7A, B). As described above, although most neurons in cluster 10 express crustacean-SIFamide, the cluster is comprised of at least two classes of neurons: OL projection neurons and AL projection neurons (Wachowiak et al. 1996; Sullivan and Beltz 2005b). These two neuronal classes have distinct structural and functional identities, OL projection neurons innervating the primary olfactory neuropil (the OL) and AL projection neurons innervating a higher-order multimodal neuropil (the AL). Establishing the final phenotypes of newborn cells in cluster 10 is an important element, therefore, in understanding the functional role of adult neurogenesis in the olfactory system. These studies showed that while the majority of cells born during the early postembryonic development of C. destructor differentiate into AL projection neurons, neurogenesis in adult crayfish is characterised by the addition of approximately equivalent numbers of both OL and AL projection neurons (Sullivan and Beltz 2005c). Adult neurogenesis, therefore, appears to play a role in the functioning of both the OL and AL in C. destructor. It remains unclear whether cells that differentiate into OL or AL projection neurons are the progeny of different progenitors or whether all newborn cells have the capacity to differentiate into either neuronal type.
In these experiments, significant numbers of double-labelled (BrdU + dextran) somata were only observed in juvenile and adult crayfish examined 4 to 6 months after exposure to BrdU, indicating that these neurons require at least 4 months to differentiate (Sullivan and Beltz 2005c), consistent with findings in P. argus (Schmidt 2001). Interestingly, similar experiments undertaken in adult crabs, C. maenas, suggest that differentiation of newborn cells in cluster 10 of these animals occurs over 3–4 weeks (Schmidt and Demuth 1998). The reasons for these inter-species differences remain unclear.
Further evidence for interspecific differences in the differentiation of newborn cells in cluster 10 has arisen from experiments in adult P. argus. In contrast to the results obtained in crayfish, Schmidt (2001) concluded that nearly all adult-born cells in cluster 10 of P. argus remain within a portion of the cluster comprised mainly of OL projection neuron somata (Wachowiak and Ache 1994; Schmidt and Ache 1996; Wachowiak et al. 1996), suggesting that most newborn cells in this species differentiate into OL projection neurons. While the functional importance of life-long neurogenesis in the olfactory pathways of decapod crustaceans remains unknown, these results suggest that its roles may differ amongst decapod taxa. Furthermore, they suggest that the AL of C. destructor may possess a functional plasticity which P. argus lacks.
Differentiation of newborn cells in the MPZ (cluster 9): local interneurons
Fully differentiated neurons within cluster 9 belong to three broad anatomical classes (OL local interneurons, AL local interneurons, OL-AL interneurons) expressing a wide array of neurotransmitters (Langworthy et al. 1997; Schmidt and Ache 1997; Schachtner et al. 2005). Pulse-chase BrdU experiments in combination with neurotransmitter immunolabelling demonstrate that newborn cells in cluster 9 of P. clarkii differentiate into neurons expressing orcokinin or allatostatin-like peptide (Sullivan et al. 2007), while those in P. argus express FMRFamide or substance P (Schmidt 2001). These double labelling experiments, however, have not yet been undertaken extensively enough to determine whether only specific neuronal types are born in this cluster and how these types might differ between species. As it has also not yet been possible to correlate the expression of particular neurotransmitters with individual interneuron classes in cluster 9, it remains unclear whether adult-born cells in cluster 9 form part of the circuitry of the OL, the AL, or of both regions.
Additional perspectives
What is a reliable and useful definition of “adult neurogenesis”?
The life-long production of new olfactory interneurons blurs the division between “developmental” and “adult” neuronal addition, and it is therefore necessary to define what is meant by “adult neurogenesis”. We believe that “adult neurogenesis” in crustaceans can be defined by proliferative systems in the brain distinct from the neuroblasts present in embryonic development. In the species we have examined (C. maenas [Beltz et al. 2005a, b], C. destructor [Fig. 6A; Sandeman and Sandeman 1998; 2000; Sullivan and Beltz 2005c], H. americanus [Fig. 6B; Harzsch et al. 1999; Benton and Beltz 2001a; Beltz et al. 2001; Beltz et al. 2007], and P. clarkii [Sullivan et al. 2007]), therefore, “adult neurogenesis” would be defined as the generation of new neurons from precursor cells with glial characteristics residing in specialised niches, rather than from neuroblasts. Such a functional definition, we believe, avoids the problems inherent in determining when an animal becomes an “adult” (Lindsey and Tropepe 2006). In rodents, for example, adult neurogenesis is considered to start after weaning (about postnatal day 21; Kempermann 2002); this is considerably before sexual maturity, a traditional measure of adulthood. Likewise, our results in decapod crustaceans suggest that adult neurogenesis in these animals commences well before sexual maturity. We are currently studying the development of the GSC and the establishment of the LPZ and MPZ to examine in detail the transition from “developmental” to “adult” neurogenesis in the olfactory pathways of these animals.
Can we generalize from data obtained in single species?
The decapod crustaceans are a very diverse group and conesequently substantial differences in adult neurogenesis could exist between taxa. It may be unwise, therefore, to consider any single species as representative of the entire group and indeed several misconceptions and misrepresentations persist in the literature on adult neurogenesis in crustaceans stemming from generalisations based on observations made on a single species. Certainly, in species of portunid crabs, freshwater crayfish and clawed lobsters, the addition of new neurons in the adult olfactory pathway is achieved by a system based on a neurogenic niche analogous to those of mammals (Figs. 3, 5, 6) (Sullivan et al. 2007). In the spiny lobster, P. argus, however, it has been reported that the generation of new neurons is based on neuroblasts that persist into adult life (Schmidt 2001, 2007a, b) and suggested that this is the general model for decapod crustaceans (Schmidt 2007a, b). The observed differences between the proliferative systems of adult P. argus and other decapods so far studied may represent real differences, in which case P. argus would appear to represent an exception amongst the decapods that have been examined thus far. Alternatively, it may be that migratory streams have yet to be observed in P. argus as the slower cell cycle in the large adults used for these studies may make this difficult. It also remains unclear whether the large cells identified as adult neuroblasts in the LPZ of P. argus are comparable to the large presumptive G2 cells located adjacent to the LPZ in the lateral migratory streams of crayfish, clawed lobsters and portunid crabs.
A misunderstanding also has arisen in the literature due to an unjustified generalisation that robust neurogenesis continues throughout life in cluster 9 only in P. argus (Schmidt 2001), despite clear and prior evidence to the contrary (Sandeman et al. 1998; Harzsch et al. 1999; Sandeman and Sandeman 2000; Benton and Beltz 2001a). Unfortunately, this particular assertion has lead to unsubstantiated evolutionary theories, claiming that the MPZ has been secondarily lost in species more recently evolved than P. argus (Lindsey and Tropepe 2006).
Next steps
The arrangement of the proliferative systems in the brains of crayfish, portunid crabs and clawed lobsters has several implications for future explorations of adult neurogenesis: (1) The fact that the glial precursor cells reside in a neurogenic niche associated with the vasculature suggests that these cells are exposed to hormones, cytokines, neurotrophins and other molecules circulating in the hemolymph. Serotonin, for instance, acts as a strong regulator of adult neurogenesis in the crustacean brain (Benton and Beltz 2001a; Beltz et al. 2001), and is most effective at very low concentrations (10−10 M; see Fig. 2D). Therefore, it may be that serotonin can act at two levels in this system: as a regulator released from central neurons directly into the proliferation zones (Beltz et al. 2001) and as a hormone regulating the cell cycle of the glial precursor cells, thus gaining direct access to the cells at the top of the neurogenic lineage. (2) This lineage appears to be organized in the migratory streams and proliferation zones in a roughly linear fashion relative to cell cycle stage, providing the exciting possibility of studying which of the cell types in the lineage are targeted by the various regulatory factors. For instance, immersing crayfish in artificial pond water with added lithium, which is known to increase levels of neurogenesis in mammalian systems (Chen et al. 2000; Kim et al. 2004), results in an accumulation of cells in the migratory streams (Fig. 8); this could suggest that the glial precursors are cycling more rapidly as a result of the lithium treatment and therefore dispatching S-phase daughters into the migratory stream at a faster rate than normal, or that migration has slowed down, resulting in clumps of cells (rather than individual cells) in the migratory streams. These alternatives can be quite readily distinguished in this system using existing technologies. The development of additional markers of differentiation for the cell types in the lineage, an approach that has been exploited successfully in mammalian systems (Kempermann et al. 2004; Steiner et al. 2006), also will provide new opportunities for defining how the various cell types are altered by regulatory factors. (3) This neuronal assembly line continues to function when the isolated brain is perfused with saline or maintained in organ culture (Benton and Beltz 2007a), providing a system that can be studied with existing connections to the brain but removed from the variables associated with whole organism studies. Finally, the neurogenic niche and glial strands also can be dissected intact from the surface of the brain (Fig. 3E), and we are currently developing short-term cultures for time-lapse imaging of the migratory streams to examine the rate of migration of daughter cells in response to environmental factors, and the self-renewal capacity of the glia-like precursors in the niche.
Fig. 8.
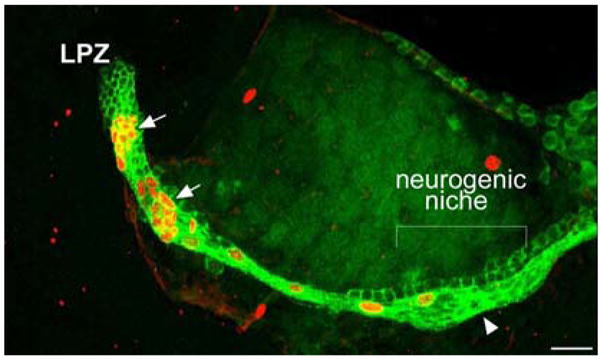
Live incubation of crayfish (P. clarkii) in 2 mM lithium chloride for seven days, followed by labelling for BrdU (orange) and glutamine synthetase (green), shows an unusual accumulation of BrdU-labelled cells along the migratory streams (arrows). Scale bar: 50 μm
Acknowledgments
This study was supported by NIH R01 MH67157, NSF IBN 0344448 and The Maren Foundation, Mount Desert Island Biological Laboratory. We thank P. Carey and G. Quinan for technical assistance, E. Buchner and Y. Yasuda for kindly providing antibodies, and S. Allodi for discussions concerning the properties of decapod glia.
References
- Ache BW, Young JM. Olfaction: diverse species, conserved principles. Neuron. 2005;48:417–430. doi: 10.1016/j.neuron.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Essential Cell Biology. 18. Vol. 17. Garland Publishing, Inc; NY: 1998. [Google Scholar]
- Allodi S, Bressan CM, Carvalho SL, Cavalcante LA. Regionally specific distribution of the binding of anti-glutamate synthetase and anti-S100 antibodies and of Datura stratonium lectin in glial domains of the optic lobe of the giant prawn. Glia. 2006;53:612–620. doi: 10.1002/glia.20317. [DOI] [PubMed] [Google Scholar]
- Arbas EA, Humphreys CJ, Ache BW. Morphology and physiological properties of interneurons in the olfactory midbrain of the crayfish. J Comp Physiol A. 1988;164:231–241. doi: 10.1007/BF00603953. [DOI] [PubMed] [Google Scholar]
- Beck RD, Jr, Wasserfall C, Ha GK, Cushman JD, Huang Z, Atkinson MA, Petitto JM. Changes in hippocampal IL-15, related cytokines, and neurogenesis in IL-2 deficient mice. Brain Res. 2005;1041:223–230. doi: 10.1016/j.brainres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Beites CL, Kawauchi S, Crocker CE, Calof A. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res. 2005;306:309–316. doi: 10.1016/j.yexcr.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Beltz BS, Sandeman DC. Regulation of life-long neurogenesis in the decapod crustacean brain. Arthropod Struct Dev. 2003;32:175–188. doi: 10.1016/S1467-8039(03)00038-0. [DOI] [PubMed] [Google Scholar]
- Beltz BS, Benton JL, Sullivan JM. Transient uptake of serotonin by newborn olfactory projection neurons may mediate their survival. PNAS. 2001;98:12730–12735. doi: 10.1073/pnas.231471298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz BS, Benton J, Sandeman DC. Adult Neurogenesis in the Crustacean Brain: Comparative Cell Cycle Dynamics and Regulatory Controls. Soc Neurosci Abstr. 2005a;31:366.2. [Google Scholar]
- Beltz BS, Benton JL, Genco MC, Mellon DeF, Sullivan JM, Sandeman DC. Regulation of adult neurogenesis in decapod crustaceans. Bull MDIBL. 2005b;44:74–77. [Google Scholar]
- Beltz BS, Tlusty MF, Benton JL, Sandeman DC. Omega-3 fatty acids upregulate adult neurogenesis. Neurosci Let. 2007;415:154–158. doi: 10.1016/j.neulet.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton J, Beltz BS. Effects of embryonic serotonin depletion on olfactory interneurons in lobsters. J Neurobiol. 2001a;46:193–205. doi: 10.1002/1097-4695(20010215)46:3<193::aid-neu1002>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Benton JL, Beltz BS. Serotonin, nitric oxide and neuronal proliferation in the olfactory pathway of lobsters. Soc Neurosci Abstr. 2001b;27:622.20. [Google Scholar]
- Benton JL, Beltz BS. An in vitro approach sheds light on serotonergic influences on adult neurogenesis in Homarus americanus. MDIBL Bull. 2007a:46. in press. [Google Scholar]
- Benton JL, Beltz BS. Nitric oxide in the crustacean brain: Regulation of life-long neurogenesis and stabilization of developing olfactory glomeruli. Dev Dynamics. 2007b doi: 10.1002/dvdy.21340. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Goy MF, Beltz BS. Nitric oxide affects serotonin levels and neuronal proliferation in the lobster olfactory pathway. Soc Neurosci Abstr. 2003;29:562.13. [Google Scholar]
- Benton JL, Beltz BS. Nitric oxide in the embryonic lobster brain: Regulation of neurogenesis and stabilization of olfactory glomeruli. Soc Neurosci Abstr. 2005;31:830.21. [Google Scholar]
- Blaustein DN, Derby CD, Simmons RB, Beall AC. Structure of the brain and medulla terminalis of the spiny lobster Panulirus argus and the crayfish Procambarus clarkii, with an emphasis on olfactory centers. J Crust Biol. 1988;8:493–519. [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourre JM, Dumont O, Piciotti M, Clement M, Chaudiere J, Bonneil M, Nalbone G, Lafont H, Pascal G, Durand G. Essentiality of omega-3 fatty acids for brain structure and function. World Rev Nutr Diet. 1991;66:103–117. doi: 10.1159/000419283. [DOI] [PubMed] [Google Scholar]
- Breithaupt T, Eger P. Urine makes the difference: chemical communication in fighting crayfish made visible. J Exp Biol. 2002;205:1221–1231. doi: 10.1242/jeb.205.9.1221. [DOI] [PubMed] [Google Scholar]
- Byrd CA, Brunjes PC. Neurogenesis in the olfactory bulb of adult zebrafish. Neuroscience. 2001;105:793–801. doi: 10.1016/s0306-4522(01)00215-9. [DOI] [PubMed] [Google Scholar]
- Campos LS. β1 integrins and neural stem cells: making sense of the extracellular environment. Bioessays. 2005;27:698–707. doi: 10.1002/bies.20256. [DOI] [PubMed] [Google Scholar]
- Carlisle DB. On the hormonal inhibition of molting in decapod Crustacea. II. Terminal anecdysis in crabs. JMBA. 1957;36:291–307. [Google Scholar]
- Chen G, Du Rajkowska GF, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- Cooper RA, Uzmann JR. Ecology of juvenile and adult Homarus. In: Cobb JS, Phillips BF, editors. The Biology and Management of Lobsters. Vol. 2. Academic Press; New York: 1980. pp. 97–142. [Google Scholar]
- Cvetic CA, Walter JC. Getting a grip on licensing: mechanism of stable Mcm2-7 loading onto replication origins. Mol Cell. 2006;21:143–144. doi: 10.1016/j.molcel.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Derby CD. Learning from spiny lobsters about chemosensory coding of mixtures. Physiol Behav. 2000;69:203–209. doi: 10.1016/s0031-9384(00)00202-x. [DOI] [PubMed] [Google Scholar]
- Derby CD, Cate HS, Steullet P, Harrison PJH. Comparison of turnover in the olfactory organ of early juvenile stage and adult Caribbean spiny lobsters. Arthropod Struct Dev. 2003;31:297–311. doi: 10.1016/S1467-8039(02)00050-6. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003a;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003b;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Cell biology of olfaction. Cambridge University Press; Cambridge: 1992. [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nature Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Garcia-Verdugo JM, Ferron S, Flames N, Collada L, Desfilis E, Font E. The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds, and mammals. Brain Res Bull. 2002;57:765–775. doi: 10.1016/s0361-9230(01)00769-9. [DOI] [PubMed] [Google Scholar]
- Goergen E, Bagay LA, Rehm K, Benton JL, Beltz BS. Circadian control of neurogenesis. J Neurobiol. 2002;53:90–95. doi: 10.1002/neu.10095. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, Monti-Graziadei GA. Continuous nerve cell renewal in the olfactory system. In: Jacobson M, editor. Development of sensory systems, vol 9. Handbook of sensory physiology. Springer; New York: 1978. pp. 55–83. [Google Scholar]
- Grünert U, Ache BW. Ultrastructure of the aesthetasc (olfactory) sensilla of the spiny lobster, Panulirus argus. Cell Tissue Res. 1988;251:95–103. [Google Scholar]
- Hallberg E, Johansson KUI, Elofsson R. The aesthetasc concept: structural variations of putative olfactory receptor cell complexes in Crustacea. Microsc Res Tech. 1992;22:325–335. doi: 10.1002/jemt.1070220403. [DOI] [PubMed] [Google Scholar]
- Hansen A, Schmidt M. Neurogenesis in the central olfactory pathway of the adult shore crab Carcinus maenas is controlled by sensory afferents. J Comp Neurol. 2001;441:223–233. doi: 10.1002/cne.1408. [DOI] [PubMed] [Google Scholar]
- Hansen A, Schmidt M. Influence of season and environment on adult neurogenesis in the central olfactory pathway of the shore crab, Carcinus maenas. Brain Res. 2004;1025:85–97. doi: 10.1016/j.brainres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Hanström B. The olfactory centres in crustaceans. J Comp Neurol. 1925;38:221–250. [Google Scholar]
- Harbige LS. Fatty acids, the immune response, and autoimmunity: a question of n-6 essentiality and the balance between n-6 and n-3. Lipids. 2003;38:323–341. doi: 10.1007/s11745-003-1067-z. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Cate HS, Swanson ES, Derby CD. Postembryonic proliferation in the spiny lobster antennular epithelium: rate of genesis of olfactory receptor neurons is dependent on moult stage. J Neurobiol. 2001;47:51–66. doi: 10.1002/neu.1015. [DOI] [PubMed] [Google Scholar]
- Hartnoll RG. The biology of Manx spider crabs. Proc Zool Lond. 1963;141:423–469. [Google Scholar]
- Hartnoll RG. Growth. In: Abele LG, editor. The Biology of Crustacea. Vol. 2. Academic Press; Orlando: 1982. pp. 111–195. [Google Scholar]
- Harzsch S. Ontogeny of the ventral nerve cord in malacostracan crustaceans: a common plan for neuronal development in Crustacea, Hexapoda and other Arthropoda? Arthropod Struct Dev. 2003;32:17–37. doi: 10.1016/S1467-8039(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Miller J, Benton J, Beltz B. From embryo to adult: persistent neurogenesis and apoptotic cell death shape the lobster deutocerebrum. J Neurosci. 1999;19:3472–3485. doi: 10.1523/JNEUROSCI.19-09-03472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. [DOI] [PubMed] [Google Scholar]
- Hinsch GW. Some factors controlling reproduction in the spider crab, Libinia emarginata. Biol Bull. 1972;143:358–366. doi: 10.2307/1540059. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Regulation of adult hippocampal neurogenesis –implications for novel theories of major depression. Bipolar Disorders. 2002;4:17–33. doi: 10.1034/j.1399-5618.2002.40101.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Adult neurogenesis: stem cells and neuronal development in the adult brain. Oxford University Press; New York: 2005. [Google Scholar]
- Kempermann G, Gage FH. New nerve cells for the adult brain. Sci Am. 1999;280:48–53. doi: 10.1038/scientificamerican0599-48. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. TINS. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kim GY. The process of establishing a social relationship influences the rate of neuronal proliferation and survival in juvenile lobsters, Homarus americanus. Wellesley College thesis 2004 [Google Scholar]
- Kim SK, Chang MY, Yu IT, Kim JH, Lee SH, Lee YS, Son H. Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J Neurochem. 2004;89:324–336. doi: 10.1046/j.1471-4159.2004.02329.x. [DOI] [PubMed] [Google Scholar]
- Langworthy K, Helluy S, Benton J, Beltz B. Amines and peptides in the brain of Homarus americanus: immunocytochemical localization patterns and implications for brain function. Cell Tissue Res. 1997;288:191–206. doi: 10.1007/s004410050806. [DOI] [PubMed] [Google Scholar]
- Lindsey BW, Tropepe V. A comparative framework for understanding the biological principles of adult neurogenesis. Prog Neurobiol. 2006;80:281–307. doi: 10.1016/j.pneurobio.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Linser PJ, Trapido-Rosenthal HG, Orona E. Glutamine synthetase is a glial-specific marker in the olfactory regions of the lobster (Panulirus argus) nervous system. Glia. 1997;20:275–283. [PubMed] [Google Scholar]
- Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28:248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Ma DK, Ming GL, Song H. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr Opin Neurobiol. 2005;15:514–520. doi: 10.1016/j.conb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon DEF, Alones V. Identification of three classes of multiglomerular, broad-spectrum neurons in the crayfish olfactory midbrain by correlated patterns of electrical activity and dendritic arborization. J Comp Physiol A. 1994;177:55–71. [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA. 2004;96:11619–11624. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Palmer TD. Adult neurogenesis and the vascular Nietzsche. Neuron. 2002;34:856–858. doi: 10.1016/s0896-6273(02)00738-9. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Sandeman D, Beltz B, Sandeman R. Crayfish brain interneurons that converge with serotonin giant cells in accessory lobe glomeruli. J Comp Neurol. 1995;352:263–279. doi: 10.1002/cne.903520209. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Denburg J. The central projections of chemoreceptor axons in the crayfish revealed by axoplasmic transport. Brain Res. 1976;115:492–496. doi: 10.1016/0006-8993(76)90365-6. [DOI] [PubMed] [Google Scholar]
- Sandeman D, Scholtz G. Ground plans, evolutionary changes, and homologies in decapod crustacean brains. In: Breitbach O, Kutsch W, editors. The nervous systems of invertebrates: an evolutionary and comparative approach. Birkhauser Verlag; Basel: 1995. pp. 329–347. [Google Scholar]
- Sandeman DC, Mellon De F. Olfactory centers in the brain of freshwater crayfish. In: Wiese K, editor. The crustacean nervous system. Springer; Berlin-Heidelberg: 2002. pp. 386–404. [Google Scholar]
- Sandeman DC, Sandeman R, Derby C, Schmidt M. Morphology of the brain of crayfish, crabs, and spiny lobsters: a common nomenclature for homologous structures. Biol Bull. 1992;183:304–326. doi: 10.2307/1542217. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Scholtz G, Sandeman RE. Brain evolution in decapod Crustacea. J Exp Zool. 1993;265:112–133. [Google Scholar]
- Sandeman R, Clarke D, Sandeman D, Manly M. Growth-related and antennular amputation-induced changes in the olfactory centers of crayfish brain. J Neurosci. 1998;18:6195–6206. doi: 10.1523/JNEUROSCI.18-16-06195.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeman R, Sandeman D. “Impoverished” and “enriched” living conditions influence the proliferation and survival of neurons in crayfish brain. J Neurobiol. 2000;45:215–226. [PubMed] [Google Scholar]
- Sandeman RE, Sandeman DC. Pre- and postembryonic development, growth and turnover of olfactory receptor neurones in crayfish antennules. J Exp Biol. 1996;199:2409–2418. doi: 10.1242/jeb.199.11.2409. [DOI] [PubMed] [Google Scholar]
- Sandeman RE, Sandeman DC. Development, growth and plasticity in the crayfish olfactory system. Microsc Res Tech. 2003;60:266–277. doi: 10.1002/jemt.10266. [DOI] [PubMed] [Google Scholar]
- Schachtner J, Schmidt M, Homberg U. Organization and evolutionary trends of primary olfactory brain centers in Tetraconata (Crustacea + Hexapoda) Arthropod Struct Dev. 2005;34:257–299. [Google Scholar]
- Schmidt M. Continuous neurogenesis in the olfactory brain of adult shore crabs, Carcinus maenas. Brain Res. 1997;762:131–143. doi: 10.1016/s0006-8993(97)00376-4. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Neuronal differentiation and long-term survival of newly generated cells in the olfactory midbrain of the adult spiny lobster, Panulirus argus. J Neurobiol. 2001;48:181–203. doi: 10.1002/neu.1050. [DOI] [PubMed] [Google Scholar]
- Schmidt M. The olfactory pathway of decapod crustaceans –An invertebrate model for life-long neurogenesis. Chem Senses. 2007a;32:365–384. doi: 10.1093/chemse/bjm008. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Identification of putative neuroblasts at the base of adult neurogenesis in the olfactory midbrain of the spiny lobster, Panulirus argus. J Comp Neurol. 2007b;503:64–84. doi: 10.1002/cne.21366. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Ache BW. Immunocytochemical analysis of glomerular regionalization and neuronal diversity in the olfactory deutocerebrum of the spiny lobster. Cell Tissue Res. 1997;287:541–562. doi: 10.1007/s004410050778. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Ache BW. Processing of antennular input in the brain of the spiny lobster, Panulirus argus. II. The olfactory pathway. J Comp Physiol A. 1996;178:579–604. [Google Scholar]
- Schmidt M, Demuth S. Neurogenesis in the central olfactory pathway of adult decapod crustaceans. Ann NY Acad Sci. 1998;30:277–280. doi: 10.1111/j.1749-6632.1998.tb10583.x. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Harzsch S. Comparative analysis of neurogenesis in the central olfactory pathway of adult decapod crustaceans by in vivo BrdU-labelling. Biol Bull. 1999;196:127–136. doi: 10.2307/1542558. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Song CK, Johnstone LM, Schmidt M, Derby CD, Edwards DH. Social domination increases neuronal survival in the brain of juvenile crayfish Procambarus clarkii. J Exp Biol. 2007;210:1311–1324. doi: 10.1242/jeb.02758. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Steiner B, Klempin F, Wang L, Kott M, Kettenmann H, Kempermann G. Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia. 2006;54:805–814. doi: 10.1002/glia.20407. [DOI] [PubMed] [Google Scholar]
- Steullet P, Cate HS, Derby CD. A spatio-temporal wave of turnover and functional maturation of olfactory receptor neurons in the spiny lobster, Panulirus argus. J Neurosci. 2000;20:3282–3294. doi: 10.1523/JNEUROSCI.20-09-03282.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld NJ, Hildebrand JG. Olfactory systems: common designs, uncommon origins? Curr Opin Neurobiol. 1999;9:634–639. doi: 10.1016/S0959-4388(99)00019-7. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz Development and connectivity of olfactory pathways in the brain of the lobster Homarus americanus. J Comp Neurol. 2001a;441:23–43. doi: 10.1002/cne.1395. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Neural pathways connecting the deutocerebrum and the lateral protocerebrum in the brains of decapod crustaceans. J Comp Neurol. 2001b;441:9–22. doi: 10.1002/cne.1394. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Evolutionary changes in the olfactory projection neuron pathways of eumalacostracan crustaceans. J Comp Neurol. 2004;470:25–38. doi: 10.1002/cne.11026. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Adult neurogenesis in the central olfactory pathway in the absence of receptor neuron turnover in Libinia emarginata. Eur J Neurosci. 2005a;22:2397–2402. doi: 10.1111/j.1460-9568.2005.04449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Integration and segregation of inputs to higher-order neuropils of the crayfish brain. J Comp Neurol. 2005b;481:118–126. doi: 10.1002/cne.20346. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Newborn cells in the adult crayfish brain differentiate into distinct neuronal types. J Neurobiol. 2005c;65:157–170. doi: 10.1002/neu.20195. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Benton JL, Beltz BS. Serotonin depletion in vivo inhibits the branching of olfactory projection neurons in the lobster deutocerebrum. J Neurosci. 2000;20:7716–7721. doi: 10.1523/JNEUROSCI.20-20-07716.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Sandeman DC, Beltz BS. Characterization of a putative stem/progenitor cell niche in the brain of an adult invertebrate, the crayfish Procambarus clarkii. Soc Neurosci Abstr. 2005;31:366.4. [Google Scholar]
- Sullivan JM, Benton JL, Sandeman DC, Beltz BS. Adult neurogenesis: a common strategy across diverse species. J Comp Neurol. 2007;500:574–584. doi: 10.1002/cne.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AJ, Thompson CS, Dunham DW. Site of pheromone reception in the crayfish Orconectes propinquus. J Crust Biol. 1984;4:554–559. [Google Scholar]
- Van der Meeren GI, Sandeman DC, Benton JL, Beltz BS. Neurogenesis and exploratory behavior in juvenile lobsters maintained in different environments. 8th International Congress of Neuroethology; 2007. in press. [Google Scholar]
- Wachowiak M, Ache BW. Morphology and physiology of multiglomerular olfactory projection neurons in the spiny lobster. J Comp Physiol A. 1994;175:35–48. [Google Scholar]
- Wachowiak M, Diebel CE, Ache BW. Functional organization of olfactory processing in the accessory lobe of the spiny lobster. J Comp Physiol A. 1996;178:211–226. [Google Scholar]
- Wahle RA, Fogarty MJ. Growth and development: understanding and modeling growth variability in lobsters Chapter 1. In: Phillips B, editor. Lobsters: biology, management, aquaculture and fisheries. Blackwell Publishing Inc; Oxford: 2006. pp. 1–44. [Google Scholar]
- Wildt M, Goergen EM, Benton JL, Sandeman DC, Beltz BS. Regulation of serotonin levels by multiple light-entrainable endogenous rhythms. J Exp Biol. 2004;207:3765–3774. doi: 10.1242/jeb.01205. [DOI] [PubMed] [Google Scholar]
- Wolff T. The maximum size of lobsters (Homarus) (Decapoda, Nephropidae) Crustaceana. 1978;34:1–14. [Google Scholar]
- Yasuda A, Yasuda-Kamatani Y, Nozaki M, Nakajima T. Identification of GYRKPPFNGSIFamide (crustacean-SIFamide) in the crayfish Procambarus clarkii by topological mass spectrometry analysis. Gen Comp Endrocrinol. 2004;135:391–400. doi: 10.1016/j.ygcen.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Yasuda-Kamatani Y, Yasuda A. Characteristic expression patterns of allatostatin-like peptide, FMRFamide-related peptide, orcokinin, tachykinin-related peptide, and SIFamide in the olfactory system of the crayfish Procambarus clarkii. J Comp Neurol. 2006;496:135–147. doi: 10.1002/cne.20903. [DOI] [PubMed] [Google Scholar]
- Yehuda S, Rabinovitz S, Mostofsky DI. Modulation of learning and neuronal membrane composition in the rat by essential fatty acid preparation: time-course analysis. Neurochem Res. 1998;23:627–634. doi: 10.1023/a:1022430620205. [DOI] [PubMed] [Google Scholar]
- Zulandt Schneider RA, Schneider RWS, Moore PA. Recognition of dominance status by chemoreception in the red swamp crayfish Procambarus clarkii. J Chem Ecol. 1999;25:781–794s. [Google Scholar]