Abstract
The phenolic acid decarboxylase gene padA is involved in the phenolic acid stress response (PASR) in gram-positive bacteria. In Lactobacillus plantarum, the padR gene encodes the negative transcriptional regulator of padA and is cotranscribed with a downstream gene, usp1, which encodes a putative universal stress protein (USP), Usp1, of unknown function. The usp1 gene is overexpressed during the PASR. However, the role and the mechanism of action of the USPs are unknown in gram-positive bacteria. Therefore, to gain insights into the role of USPs in the PASR; (i) a usp1 deletion mutant was constructed; (ii) the two genes padR and usp1 were coexpressed with padA under its own promoter as a reporter gene in Escherichia coli; and (iii) molecular in vitro interactions between the PadR, Usp1, and the padA promoter were studied. Although the usp1 mutant strain retained phenolic acid-dependent PAD activity, it displayed a greater sensitivity to strong acidic conditions compared to that of the wild-type strain. PadR cannot be inactivated directly by phenolic acid in E. coli recombinant cultures but is inactivated by Usp1 when the two proteins are coexpressed in E. coli. The PadR inactivation observed in recombinant E. coli cells was supported by electrophoretic mobility shift assays. Although Usp1 seems not to be absolutely required for the PASR, its capacity to inactivate PadR indicates that it could serve as an important mediator in acid stress response mechanisms through its capacity to interact with transcriptional regulators.
Phenolic acids are essential plant compounds involved in the molecular bonds between cellulose, hemicellulose, and lignin in the cell wall. They are significant in the mammalian diet (32). Their specific structure confers important biological activities. They have free radical scavenging properties that can trigger antimutagenetic effects (14) but that can also lead to pro-oxidant DNA degradation mediated by divalent cations (37). Recently, it was shown that some phenolic acids that induce the phenolic acid stress response (PASR) in gram-positive bacteria regulate the expression of genes of the type III secretion system required for virulence by the plant pathogen bacterium Dickeya dadantii (23). Phenolic acids such as p-coumaric, ferulic, and caffeic acids are toxic for numerous gram-positive bacteria such as Bacillus subtilis (33), Pediococcus pentosaceus (4), and Lactobacillus plantarum (16) under acidic condition. Phenolic acid decarboxylase (PAD) activity in these bacteria is a detoxifying system specifically and strongly induced by these chemicals. Two genes involved in the PASR have been characterized: padA and padR. The padA gene (named padC in B. subtilis) encodes the PadA enzyme and padR encodes the PadR transcriptional repressor. Deletion of padA leads to growth inhibition in the presence of phenolic acids, especially at low pH (3), while deletion of padR leads to constitutive overexpression of padA (16) and, consequently, to high resistance to phenolic acids. Although the PadR-like proteins constitute a regulatory family of more than 1350 putative members in the bacterial genome database (accession no. Pfam PF03551), only a few of them are currently under investigation. Among them, the best characterized is AphA, the repressor of the penicillin amidase gene (21) and the transcriptional activator of the virulence cascade of Vibrio cholerae, which acts in consortium with the coactivator AphB (20). Another member of this family, LmrR, acts as a repressor of the multidrug recognition operon lmrCD in Lactococcus lactis, and its molecular structure in the presence or absence of H33342 or daunomycin drugs has been analyzed (26). As well as for the other PadR like proteins family, the way by which PadR is inactivated remains also poorly elucidated. Concerning the PASR, we have previously demonstrated that in growing cultures of a recombinant Escherichia coli strain expressing PadR from P. pentosaceus and in L. plantarum, PadR could not be inactivated by exogenously added phenolic acids, even when the expression of padR was low (4, 16). We recently showed that partial inactivation of PadR was possible by the addition of phenolic acid at the onset of growth of a recombinant E. coli strain expressing the padC and padR genes from B. subtilis (33). However, this inactivation was very limited compared to that observed in phenolic acid-induced B. subtilis cultures. In L. plantarum, padA and padR form a divergon, and padR was predicted to be coexpressed with usp1, whose product displays significant identity with proteins belonging to the universal stress protein (USP) family (16). Expression of the putative bicistronic operon padR-usp1 is induced by the addition of phenolic acids to the culture medium (24). Taken together, these data suggest that Usp1 might be a mediator of PASR by inactivating PadR, or a putative transcriptional activator of padA, which could enter in competition with PadR for its promoter. In order to study the role of usp1 in PadR inactivation, we deleted the usp1 gene from the wild-type L. plantarum strain or coexpressed it with padR and padA in E. coli, a species devoid of the PASR. Although the usp1 deletion mutant still retained inducible PAD activity, we demonstrate that Usp1 inactivates PadR in recombinant E. coli strains. These results were confirmed by electrophoretic mobility shift assays (EMSAs) with a padA promoter probe and PadR. We also demonstrate that Usp1 is involved in the acid stress response in cells entering into stationary growth phase.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in the present study are listed in Table 1. L. plantarum LPNC8 strain, kindly given by Lars Axelsson (Matforsk, Osloveien, Norway), was routinely grown in MRS agar or liquid medium (9) at 37°C without shaking, and E. coli was grown aerobically in Luria-Bertani (LB) medium at 37°C. The growth of bacteria was evaluated by monitoring the absorbance of culture at 600 nm with the appropriate dilution. Antibiotics were used in the following concentrations in the corresponding media: ampicillin, 200 μg/ml; erythromycin, 100 μg/ml; and kanamycin, 50 μg/ml for E. coli and 5 μg of erythromycin/ml for L. plantarum. To test bacterial resistance to acid shock, 100-μl samples of bacteria grown in MRS medium at an initial pH of 6.5 at 37°C were diluted 100-fold in a 10-ml volume of MRS medium with a pH 2.5. Cells were incubated for 1 h at 37°C. Viability was determined by CFU counts of culture dilutions plated onto MRS agar plates.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| TG1 | supE hsdΔ5thi Δ(lac-proAB)F′ [traD36 proAB+ lacIqlacZΔM15] | 15 |
| BL21(DE3) Star | F−ompT hsdSB (rB− mB−) gal dcm (DE3) | Invitrogen |
| TOP 10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 deoR araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| L. plantarum | ||
| NC8 (wild type) | Wild type, gram-positive ubiquitous homolactic acid bacterium, plasmid-free strain | 2 |
| LPNC8ΔUSP1 (Δusp1) | L. plantarum NC8 strain with usp1 disrupted by double homologous recombination | This study (Fig. 3) |
| LPNC8ΔPADR (ΔpadR) | L. plantarum NC8 strain with padR disrupted by double homologous recombination | 16; Fig. 3 |
| Plasmids | ||
| pTZ19R | Ampr; ΔlacZ | Novagen |
| pET28a+ | Kanr; vector for overexpression of His-tagged proteins using the T7 bacteriophage promoter | Novagen |
| TOPO PCR 2.1 | Ampr Kanr; ΔlacZ, vector for TA cloning | Invitrogen |
| pGID023 | Shuttle vector for E. coli and L. plantarum; derivative of pJDC9 containing the pE194 replication functions; used as an unstable integration vector; Ermr | 18 |
| pJPDC1 | Emr; pJDC9 containing the 2.3-kbp Sau3A fragment of L. plantarum corresponding to the locus with padA-padR genes | 6; Fig. 4 |
| pLOCPAD | Ampr Kanr; TOPO vector containing the 2.212-kbp LOCPAD fragment with padA, padR, and usp1 PCR amplified with primers LPLOC1 and LPLOC2 | This study (Fig. 4) |
| pTD14 | Ampr; pTZ19R containing the 311- and 385-bp DNA fragments PCR amplified with the primers LPDELU1-LPDELU2 and LPDELU3-LPDELU4, respectively | This study |
| pGΔUSP1 | Emr; pGID023 containing the 694-bp D14 fragment PCR amplified with the primers LPDELU1 and LPDELU4 | This study |
| pED | pET28a+ with padA under its own promoter cloned into SphI restriction site | This study (Fig. 4) |
| pER | pET28a+ containing padR between NcoI and XhoI sites to produce PadR | 16; Fig. 4 |
| pEU | pET28a+ containing usp1 between NcoI and XhoI sites to produce Usp1 | This study (Fig. 4) |
| pEDR | pER with padA under its own promoter cloned into SphI restriction site | This study (Fig. 4) |
| pERU | pET28a+ with padR and usp1 cloned between NcoI and XhoI restriction sites | This study (Fig. 4) |
| pEDRU | pERU with padA under its own promoter cloned into SphI restriction site | This study (Fig. 4) |
Emr, erythromycin resistance; Ampr, ampicillin resistance; Kanr, kanamycin resistance.
DNA manipulation, PCR amplification, and transformation procedures.
DNA manipulation, purification, ligation, restriction analysis, and gel electrophoresis were carried out as described by Sambrook et al. (31). L. plantarum chromosomal DNA was prepared by the method described by Posno et al. (30). PCR was performed with 0.1 U of Taq DNA polymerase (MP Medicals), according to the manufacturer's recommendations, in an automatic thermocycler (Bio-Rad). Custom primers (Eurogentec) are listed in Table 2. PCR, and restriction products were purified by using a QIAquick PCR purification kit or a QIAgel agarose gel extraction kit (Qiagen). E. coli and L. plantarum strains were transformed by electroporation as described by Dower et al. (12) and Aukrust and Nes (1), respectively. E. coli BL21(DE3) Star was transformed by the CaCl2 procedure (7).
TABLE 2.
Primers
| Function and primer | Sequence (5′→3′) | Site createda |
|---|---|---|
| Probe synthesis | ||
| LPD1 | ACCGACACTGATCCACTCAT | |
| LPD2 | CACCGATCTCGTCATCAAACG | |
| LPD3 | CACCGATCTCGTCATCAAACG | |
| LPD4 | GTCTAATATGTCGTTTTAATC | |
| LPD5 | GACTGCAGGGCGACCGTTTTCCCGCAAGC | PstI |
| LPU1 | AATGACGTCATCCACATCAC | |
| LPU2 | TCAGAACGTGCGTTTCGATA | |
| LPU3 | GTCGAAGATCCAAGCTAAAC | |
| Deletion of usp1 | ||
| LPDELU1 | GATAAGCTTTATAGCCATCCTTGCT | HindIII |
| LPDELU2 | GTTCTGCAGTTCTAAGATTGCTGG | PstI |
| LPDELU3 | TTGCTGCAGGATAGACAAGTGGCT | PstI |
| LPDELU4 | AACGGATCCAGTTAGCACACTTAC | BamHI |
| Plasmid construction | ||
| LPDLOC1 | AATGAACAATAGCAGTCAAAACAA | |
| LPDLOC2 | ACGTTTGTCCTACCACCACATTT | |
| LPD6 | GGAGCATGCTAATGGTTGCTGGTTTA | SphI |
| LPD7 | GGAGCATGCACGTTTGTCCTACCAC | SphI |
| LPU4 | AAGCCATGGAAAATCAAAAAATGC | NcoI |
| LPU5 | AATCTCGAGTTACCGAACAACGAT | XhoI |
| LPR1 | CCCATGGCGCAAAAAAATAAGTTACAA | NcoI |
The sites are underlined in column 2.
Whole-RNA extraction.
Cells from a 20-ml culture of L. plantarum were harvested and washed in cold ultrapure water by centrifugation and immediately resuspended in 1 ml of Tri-Reagent (Sigma) containing 100 mg of 50- to 70-μm glass beads. Total RNA was extracted immediately by disrupting the cells using a FastPrep System (MP Medicals). After this step, the procedure for total RNA purification was carried out according to the guidelines of the Tri-Reagent manufacturer. DNA traces were eliminated by incubating samples at 37°C for 40 min with 3 U of DNase I (MBI Fermentas). This enzyme was then inactivated for 10 min at 65°C. The effectiveness of the DNase treatment was checked by the absence of amplicons in PCR amplification of samples.
Southern and Northern blot analyses.
The Southern blot was carried out with the usp1 DNA probe on the L. plantarum genomic DNA digested with EcoRI. For Northern blotting, total RNA were resolved by denaturing formaldehyde agarose gel electrophoresis and transferred to nylon membranes (Nytran; Schleicher & Schuell) by using the Pharmacia vacuum system. padR- and usp1-specific probes were PCR amplified with the primer pairs LPD1/LPD2 and LPU1/LPU2, respectively, designed from L. plantarum NC8 chromosomal DNA. After purification, the DNA probe was labeled with [α-32P]dATP (3,000 Ci/mmol; Perkin-Elmer) by random priming (Invitrogen). Transcript size was determined by comparison with an RNA ladder (0.24 to 9.5 kb; Invitrogen).
Plasmid constructs.
All of the constructs used in the present study are presented in Fig. 3. Plasmid pLOCPAD was obtained by cloning into the vector TOPO TA (Invitrogen) the whole PCR-amplified (with the primers LPDLOC1 and LPDLOC2) 2.212-kbp padA DNA locus, including padA, padR, and usp1 under their own promoters and transcriptional terminators. The pEDR plasmid was constructed by inserting the 979-bp padA fragment into the SphI restriction site of plasmid pER (16). The padA DNA fragment encompassing the first 60-bp of padR gene (16), the padA coding sequence, and the 267-bp downstream the TAA stop codon containing the transcriptional terminator was PCR amplified with the primers LPD6 and LPD7. Plasmids pEU and pERU were obtained by inserting the NcoI-XhoI DNA fragments corresponding, respectively, to the usp1 coding sequence and the padR and the usp1 coding sequences between the NcoI and XhoI sites of pET28a+. The DNA fragments were PCR amplified with the primers LPU4 and LPU5 for usp1 and the primers LPR1 and LPR2 for the operon padR-usp1. The TAA stop codon of usp1 was conserved to produce a native Usp1 protein without His tag. The plasmid pEDRU was derived from pERU by inserting the padA DNA fragment into the SphI restriction site (see the details above). The padR gene or the padR-usp1 operon was cloned under the control of the T7 promoter of vector pET28a+, and resulting plasmids were transformed into E. coli BL21(DE3) Star (Invitrogen).
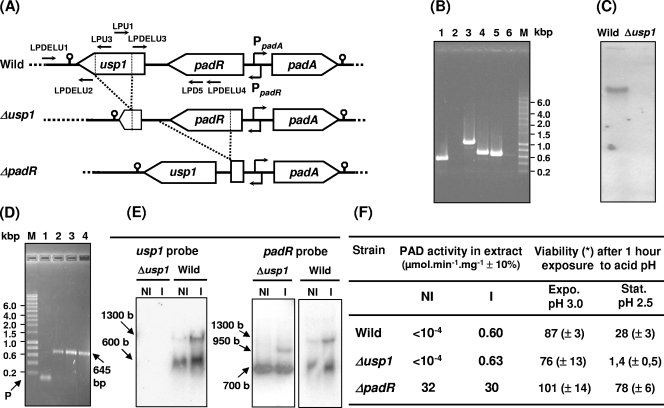
FIG. 3.
Genetic and phenotypic comparison of wild-type and mutant strains. (A) padA gene locus organization in the strains. PpadA and PpadR are the overlapping diverging promoters of padA and padR, respectively. Small horizontal arrows indicate primers for PCR amplification used in panels B, C, and D experiments. (B) Amplicon from chromosomal DNA of the usp1 region with three primer pairs (see panel A) on wild-type strain (lanes 1, 3, and 5) and Δusp1 mutant strain (lanes 2, 4, and 6). Lanes 1 and 2, amplicon with primers LPDELU1 and LPU3; lanes 3 and 4, amplicon with the primers LPDELU1 and LPD5; lanes 5 and 6, amplicon with the primers LPU1 and LPDELU4. (C) Southern blot with usp1 probe on digested DNA from wild-type and Δusp1 strains. (D) RT-PCR with the primers LPD5 and LPU1, which bind within the padR and the usp1 genes, respectively, and RNA extract from noninduced (lane 2) and p-coumaric acid-induced (lane 3) cultures of L. plantarum: lane 1, PCR control without the RT step to validate DNase treatment; lane 4, control PCR with DNA from L. plantarum; P, primer control (no amplification); lane M, molecular mass marker (Smart Ladder; Eurogentec). (E) Northern blot with total RNA from noninduced (NI) and 1.2 mM p-coumaric acid-induced (I) bacteria of Δusp1 mutant and wild-type strains. (F) Phenotypes of the strains: PAD activity in either noninduced (NI) and 1.2 mM p-coumaric acid, 10-min induced (I) strains and viability of cells from exponential (Expo.) or early stationary (Stat.) stage of growth after 1 h of exposure to pH 3.0 and 2.5. The results are an average of three independent experiments. Standard deviations are shown in parentheses.
Knockout of usp1.
The plasmid pTD14, which carries usp1 with a deletion of a 352-bp fragment between 37 bp downstream of the start codon and 73 bp upstream of the stop codon, was constructed by simultaneously cloning two separate DNA fragments between HindIII and BamHI sites of pTZ19R. Primers LPDELU1 and LPDELU2, which include an HindIII and a PstI site, respectively, were used to amplify the 311-bp region encompassing the last 73 bp of usp1, and the 238-bp region downstream of usp1, which contains the transcriptional terminator of the putative padR-usp1 operon (Fig. 3A) (16). The 385-bp DNA fragment encompassing the 157-bp upstream of the stop codon of padR, the 190-bp region between the stop codon of padR and the ATG codon of usp1, and the first 37 bp of the 5′ end of usp1 were PCR amplified with the primers LPDELU3 and LPDELU4, which include PstI and BamHI restriction sites, respectively. The 575-bp fragment encompassing the two 311- and 385-bp fragments were PCR amplified from plasmid pTD14 with primers LPDELU1 and LPDELU4 and cloned between the HindIII and BamHI sites of the vector pGID023 to obtain plasmid pGΔUSP1 (Table 1). This plasmid was transformed into L. plantarum NC8, and transformants were selected on MRS medium containing 5 μg of erythromycin and 12 μg of lincomycin/ml. A few colonies were grown individually without antibiotics to produce 50 generations, and a diluted sample of each culture was poured onto MRS solid medium also without antibiotics. About 20 colonies were tested for simultaneous resistance to erythromycin and lincomycin. Five sensitive colonies were then screened by PCR amplification, with appropriate primers, to select clones giving the expected usp1 gene deletion (Fig. 3A and B). Sequencing (Cogenics, France) of the amplicon obtained with the primers LPDELU1 and LPDELU4 was performed to confirm the deletion.
Cell extracts, assays for PAD activity, protein purification, and electrophoresis.
L. plantarum and recombinant E. coli strain cultures, as well as cell disruptions, were performed as previously described (3) by using a Z Plus series cell disrupter (Constant System) (two passages at 108 Pa). The PAD activity in the remaining whole cells and in cell extracts was measured as previously described (3), which consists of monitoring the disappearance of absorption peaks of the phenolic acid substrates and the simultaneous appearance of new peaks corresponding to the vinyl derivatives by UV spectrophotometry. The protein concentration in cell extracts was determined by using a protein assay kit (Bio-Rad) with bovine serum albumin as a standard. For overexpression of PadR and Usp1 proteins, 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added to the appropriate cultures of E. coli at the mid-exponential growth phase, and incubation was continued overnight at 37°C. Cells were harvested by centrifugation (4,000 × g), washed with saline, suspended in 25 mM phosphate buffer (pH 8) to 2% of the initial culture volume, and disrupted with the Z Plus series cell disrupter. His-tagged PadR protein was purified by overexpression and purification over a 0.5-ml nickel-nitrilotriacetate (Ni-NTA) agarose column (Qiagen). The PadR protein was eluted with an imidazole gradient of 20 to 100 mM. Protein extracts were resolved by denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with a 12.5% polyacrylamide resolving gel, as previously described (16). To study the interaction between PadR and Usp1, extracts from recombinant E. coli expressing separately or simultaneously the two proteins were mixed together and resolved in 10% (wt/vol) PAGE (60 V, 10 h) without SDS denaturation or heating.
EMSAs.
The 189-bp DNA probe corresponding to the promoter region of padA was PCR amplified with primers LPD3 and LPD4 and labeled with T4 polynucleotide kinase (Invitrogen) in the presence of [γ-32P]ATP (Perkin-Elmer). Standard EMSAs were performed as follows. Purified PadR (from 2 to 20 nM) was incubated for 20 min at 28°C in 15 μl of binding buffer containing 10 mM Tris-HCl (pH 7.8), 5% (vol/vol) glycerol, 0.2 mM EDTA, 50 mM KCl, 2 mM MgCl2, 2 mM dithiothreitol, 2.5 μg of bovine serum albumin/ml as unspecific protein competitor, and 2.5 μg of salmon sperm DNA/ml as an unspecific DNA competitor. To verify the specificity of the binding of PadR to the padA promoter, 50- and 200-fold excesses of specific unlabeled padA promoter fragment or a 1,000-fold excess of unspecific competing poly(dI-dC) was tested as previously described (33). The samples were resolved onto 5% (wt/vol) PAGE gels, which were dried and analyzed by autoradiography. For testing the effect of phenolic acids on the binding, PadR was first incubated with different concentrations of p-coumaric acid in 15 μl of binding buffer at room temperature for 15 min. The probe was then added in the above mixture and incubated for 20 min at 28°C before loading in a 5% polyacrylamide gel. For testing the effect of Usp1 on PadR, the protein extract from the Usp1 overexpressing E. coli pEU (U) strain, where Usp1 represents ca. 90% of the total proteins, was used. An extract of the E. coli strain with the vector pET28a+ without usp1 was used as a negative control.
Glutaraldehyde cross-linking of PadR and Usp1.
A modified procedure of Derré et al. (10), described by Gury et al. (16), was used for the cross-linking of PadR and Usp1. Glutaraldehyde was used at concentrations ranging from 0.25 to 1% (vol/vol). The samples were heated with loading buffer and analyzed by SDS-PAGE on a 12% (wt/vol) gel. The gel was stained with Coomassie blue.
RESULTS
Nucleotide and protein sequence analysis of the padA/padR-usp1 locus.
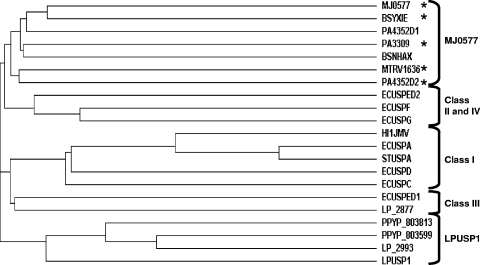
To clone the complete LPNC8 sequence downstream of usp1, an inverse PCR amplification strategy was used (data not shown). This sequence was unknown at the time the present study was performed because the genome sequence of the L. plantarum WCFS1 strain had not yet been published. Analysis of the 462-bp sequence of usp1 (AJ289188) revealed that the deduced product of 154 amino acid residues displayed 100% identity with the putative LP_3663 protein in the subsequent available genome of L. plantarum WCFS1 (http://cmr.tigr.org/CMR/Search.shtml) (19). LP_3663 is 1 of the 10 putative USPs of unknown function that are found in the WCFS1 strain. The paralogs that displayed the more significant identity with Usp1 in the WCFS1 strain are LP_2877 (36%), LP_2993 (33%), and LP_1163 (24%) (Fig. 1). Identity with the seven other L. plantarum putative USPs is below 20%. Multiple alignment of the USP amino acid sequences revealed that Usp1 displays higher identity with putative USPs of lactic acid bacteria belonging to the genus Lactobacillus (L. brevis, L. sakei, L. casei, and L. salivarius) but also with Pedicoccus spp. and Oenococcus oeni (Fig. 1). The alignment revealed that these proteins can effectively be classified as members of the USP family (Pfam PF00582 or COG0589). They possess the ATP binding domain (G-2X-G-9X-G-S/T) carried by the Methanococcus jannaschii MJ0577 Usp (36), but in a weakly conserved form. It is interesting that all of these species share many of the natural habitats of L. plantarum and that P. pentosaceus, the other lactic acid bacterium in which the PASR was studied, possesses two high similar Usp1 homologues (PPYP_803599 and PPYP_803813). Phylogenetic analysis of USPs primary sequences shows that PPYP_803599, PPYP_803813, LP2993, and Usp1 form a distinct subfamily (27) (Fig. 2).
FIG. 1.
Amino acid sequence alignment of Usp1 with homologous proteins from GenBank and MJ0577. Alignment was achieved by using online software (http://bioinfo.genopole-toulouse.prd.fr/multalin/multalin.html; version 5.4.1) (8). The protein origin and the GenBank codes are indicated (LP, L. plantarum; LVIS, L. brevis; LSEI, L. casei; LSA L. sakei; PPYP, P. pentosaceus; OENOO, O. oeni; MJ, M. jannaschii). The consensus sequence of USP is also shown (capital letters correspond to conserved residues). Symbol comparison table: blosum62. Consensus symbols: !, anyone of IV; %, anyone of FY; #, anyone of NDQEBZ. The sizes and the global identity percentages between these sequences and Usp1 are indicated on the right. Residues of the ATP binding domain are boxed, and regions predicted to contact the adenosine (A), ribose (R), or triphosphate (P) are marked.
FIG. 2.
Phylogenetic tree of USP. USPs are classified according to the method of Nachin et al. (27). Bacterium species and protein code in GenBank are indicated (MJ, M. jannaschii; BS, B. subtilis; PA, Pseudomonas aeruginosa; MT, Mycobacterium tuberculosis; EC, E. coli; HI, Haemophilus influenzae; ST, Salmonella enterica serovar Typhimurium; LP, L. plantarum; PP, P. pentosaceus). Proteins carrying the conserved MJ0577 ATP-binding domain are marked with an asterisk. This tree was produced using CLUSTAL W software (22a).
Transcriptional analysis of padA, padR, and usp1 in L. plantarum.
Sequence analysis (http://www.softberry.com) of the padR-usp1 intergenic region (Fig. 3A) did not detect the presence of a typical transcription terminator for padR. Moreover, our attempts to identify a putative usp1 initiation transcription site by primer extension were unsuccessful, both with RNA extract from noninduced and p-coumaric-acid induced bacteria (data not shown). For Usp1, a strong transcription terminator (ΔG = −27 kcal mol−1) was found 46 nucleotides downstream of the usp1 stop codon. Downstream of this terminator we found the promoter of the adhE gene, which encodes a putative bifunctional alcohol and acetaldehyde dehydrogenase, a gene also found in the same locus in the strain WCFS1 (lp_3662). Northern blot hybridization was performed using RNA samples from cultures of L. plantarum, with or without induction with p-coumaric acid as templates, and the padR and the usp1 DNA probes (Fig. 3E). Two transcripts were observed with the two probes. The two bands of about 700 and 600 bases correspond to the size of the single transcript of padR and usp1, respectively. The 1,300-base band, which appears less intense than the 700- and 600-base bands, corresponds to the size of the padR-usp1 cotranscript.
Since no usp1 promoter and no padR transcription terminator could be identified in the intergenic region, the single transcripts could result from cleavage of the cotranscript. These results are in agreement with those previously reported using quantitative reverse transcription-PCR (RT-PCR), where after addition of p-coumaric acid, the padR and usp1 relative transcript levels (RTLs) followed the same kinetics of expression as that of padA, but with lower folds of induction (37 and 13, respectively) compared to about 8,000 for padA (24). Unlike usp1, the expression of the three most similar paralogous genes in L. plantarum WCFS1 measured by quantitative RT-PCR in similar conditions was not induced by p-coumaric acid (data not shown), suggesting that these USPs have different functions in this bacterium.
Knockout of usp1 and phenotypic characterization of the mutant strain.
To study the putative role of Usp1 in the PASR, usp1 was interrupted by deletion of an internal region corresponding to the putative ATP binding motif (for details, see Materials and Methods and Fig. 1 and 3A). Among the few putative mutants obtained after the Campbell double recombination event, the mutant, named LPNC8ΔUSP1 (Δusp1), gave the expected amplicon size profile with selected primers (Fig. 3B) and was retained for further study. Southern blot with a DNA probe corresponding to the deleted fragment of usp1 confirmed the presence of a deletion in the usp1 sequence (Fig. 3C). A Northern blot analysis carried out with the same probe on RNA extracts from noninduced and p-coumaric acid-induced Δusp1 cultures gave no usp1 transcript (Fig. 3E), whereas the result with the padR probe indicated that the deletion of usp1 did not affect padR expression, a condition essential to study the effect of the usp1 deletion on PadR. In the Δusp1mutant, the smaller size of the padR-usp1 cotranscript of ∼950 bases results from the 350-bp deletion in the usp1 gene. Cultures of the wild-type or the Δusp1 mutant strain were induced at an optical density at 600 nm of 0.6 by the addition of 1.2 mM p-coumaric acid, and the PAD activity was tested in corresponding cell extracts (Fig. 3F). No significant difference in PAD activity was observed between the two strains, indicating that Usp1 was not absolutely required for PadR inactivation by p-coumaric acid.
The ability of the Δusp1 mutant to survive after exposure to different stresses was compared to that of the wild-type and the ΔpadR mutant strain, in which Usp1 was overexpressed as previously demonstrated (24). These strains markedly differed in their ability to survive acid stress shock at pH 2.5 applied for 1 h at the onset of stationary growth phase (Fig. 3F). In these conditions, viability of the Δusp1 mutant strain was dramatically reduced and about threefold increased in the ΔpadR strain when Usp1 was constitutively expressed. These results indicate that Usp1 is involved in the global acid stress response in this bacterium.
Expression of the padA locus genes in E. coli.
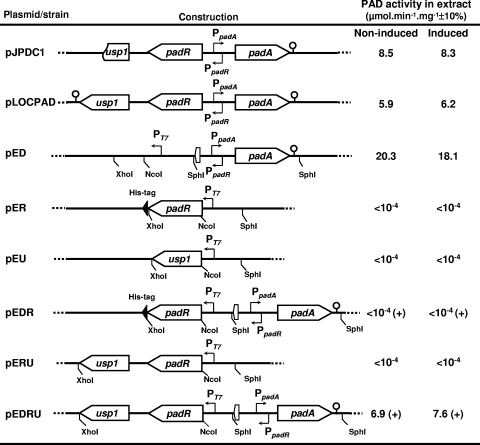
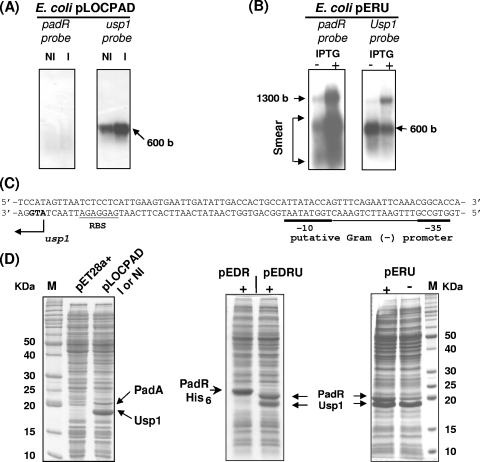
The USP family has never been characterized in L. plantarum or in other lactic acid bacteria. Therefore, we introduced a functional padA locus in E. coli, which is devoid of the PASR genes, to avoid any possible interference with other genes from the host L. plantarum and to explore the possible interaction between the coexpressed proteins Usp1 and PadR. The complete DNA locus encompassing the padA/padR-usp1 genes was PCR amplified and cloned into the vector TOPO PCR 2.1 to obtain the recombinant E. coli strain pLOCPAD (Fig. 4). Crude extracts from E. coli pLOCPAD, in the presence or absence of p-coumaric acid, exhibited a constitutive PAD activity of about 6 μmol min−1 mg−1, a level similar to that of the E. coli pJPDC1 clone isolated from an L. plantarum genomic library in which usp1 was truncated (6). In this recombinant E. coli strain, no padR mRNA was detected by Northern blot analysis with RNA extracted from cells with or without p-coumaric acid induction (Fig. 5A). However, the use of the usp1 DNA probe revealed a single transcript of about 600 bp. The corresponding SDS-PAGE patterns were identical in both inducing and noninducing conditions (Fig. 5D), and two bands corresponding to Usp1 and PadA were observed. These results suggest that the E. coli RNA polymerase could not bind to the padR promoter that overlaps divergently with the padA promoter but indicates that the padA promoter was functional in E. coli. Since PadR was not synthesized in this strain, padA was constitutively transcribed. In E. coli pLOCPAD, usp1 seemed to be expressed from an intermediate putative promoter, which was recognized by the RNA polymerase from E. coli. This hypothesis was reinforced by sequence analysis (Softberry, Inc., Mount Kisco, NY) of the padR-usp1 intergenic region that allowed us to identify a putative Sigma 70-like promoter for E. coli in this region (Fig. 5C).
FIG. 4.
Physical map of the different plasmid constructs of padA, padR, and usp1 and the resulting PAD activity obtained from the host strains. Plasmids pJPDC1 and pLOCPAD were constructed with the pJDC9 and the TOPO PCR 2.1 vectors, respectively. Plasmids pED, pER, pEU1, pEDR, pERU, and pEDRU were constructed by using the pET28a+ vector. Closed triangles represent His tag fusions. Promoters are indicated by small arrows at the −35 position. Restriction sites used for construction are indicated. For PAD activity, I and NI correspond to protein extract from cells either induced (I) or not induced (NI) with 1.2 mM p-coumaric acid. (+), Induction with IPTG.
FIG. 5.
Transcriptional analysis of padR and usp1 in recombinant E. coli strains and corresponding SDS-PAGE of protein extracts. (A) Northern blot with RNA extract from noninduced (NI) and 1.2 mM p-coumaric acid 10-min-induced (I) cells. (B) Northern blot with RNA extract from 1 mM IPTG-induced (+) or not induced (−) cells. The 600- and 1,300-base bands correspond to the usp1 and padR-usp1 transcripts, respectively, while “Smear” probably represents degradative products from the 1,300-base transcript. (C) DNA sequence upstream of usp1 indicating the −35 and −10 boxes that could serve as a promoter for usp1 in the recombinant E. coli strains pLOCPAD and pERU. (D) SDS-PAGE analysis of crude protein extracts from the recombinant E. coli strains. pET28a+, E. coli TG1 with the vector pET28a+; I, 1.2 mM p-coumaric acid-induced cell extracts; - and +, noninduced (−) and 1 mM IPTG-induced (+) cell extract. M, molecular mass standards.
Coexpression of padR-usp1 genes in E. coli.
To overcome the lack of padR expression inthe padA locus in E. coli, the padR-usp1 operon was cloned under the control of the pET28a+ T7 promoter (Fig. 4). SDS-PAGE analysis of total cell extracts from E. coli pERU was carried out in the presence or absence of 1 mM IPTG (Fig. 5D). In noninducing conditions, two bands of about 21 and 18 kDa, corresponding to the molecular masses of PadR and Usp1, respectively, were observed, but the concentration of PadR appeared slightly lower than that of Usp1. This result is in agreement with the hypothesis of a putative usp1 promoter being recognized in E. coli. With 1 mM IPTG induction, the two proteins were produced at approximately the same level. Northern blot analysis of total RNA from E. coli pERU was performed under inducing or noninducing conditions, with the padR and usp1 DNA probes (Fig. 5B). As previously observed with total RNA from L. plantarum, two bands of about 600- and 1,300-base were detected with the usp1 DNA probe. With the padR probe, the 1,300-base cotranscript was strongly produced. In this sample, a smear of mRNA of lower molecular mass may represent degradative products of the 1,300-base transcript. The cotranscription of padR and usp1 in E. coli was confirmed by RT-PCR amplification experiments with mRNA prepared from E. coli pERU cells under inducing or noninducing conditions (1 mM IPTG) and the primers LPD5 and LPDU1 (Fig. 3A and D).
Expression of usp1 in E. coli inactivates the PadR repressor.
To investigate the involvement of usp1 in the PASR, padR and usp1 genes were cloned individually or together in the pET28a+ vector under the control of the T7 promoter. In addition, padA was cloned under its own promoter into the SphI site of the pERU plasmid to serve as a reporter gene (Fig. 4). Control E. coli BL21 pER and E. coli BL21 pERU strains without padA did not display PAD activity. In E. coli BL21, the T7 promoter was operational to express the operon padR-usp1. SDS-PAGE analysis of crude extracts from E. coli BL21 pEDR and E. coli BL21 pEDRU showed that PadR was either produced at a low level without induction (data not shown) or overproduced with IPTG induction (Fig. 5D) and that Usp1 was well produced even without IPTG induction (Fig. 5D). This is in agreement with bioinformatics analysis, which revealed that the padR-usp1 intergenic region could serve as promoter in E. coli. No PAD activity was detected in either noninduced or p-coumaric acid-induced extracts from E. coli pEDR cells (Fig. 4). In contrast, crude extracts of E. coli BL21 pEDRU, in which PadR and Usp1 were produced at nearly the same concentrations (Fig. 5D), exhibited a constitutive PAD activity of ∼7 μmol min−1 mg−1 either with or without induction with p-coumaric acid (Fig. 4). This heterologous expression of the three genes of the L. plantarum padA locus in E. coli demonstrates that Usp1 is able to inactivate the PadR repressor when it is produced at near the same level in the bacteria.
Usp1 abolishes the binding of PadR with the padA promoter.
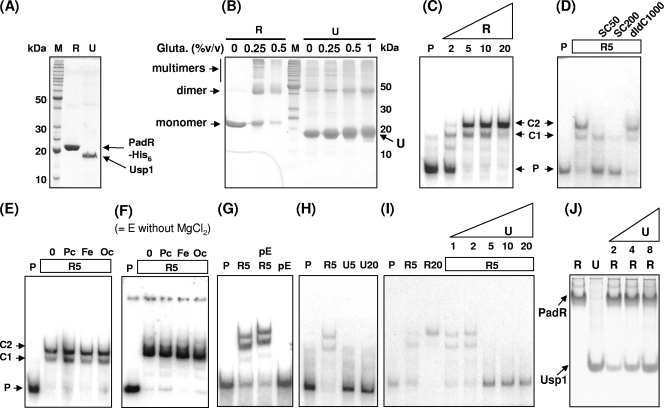
Mobility shift assays were carried out to study the influence of Usp1 on the ability of PadR to bind to the padA promoter. The PadR-His6 and Usp1 proteins were overproduced in pER and pEU recombinant E. coli strains, respectively (Fig. 4 and Fig. 6A) and used in different EMSAs involving different combinations or treatments and with the appropriate controls (Fig. 6). Before carrying out EMSA experiments, the capacity of these two proteins to dimerize was tested by glutaraldehyde cross-linking experiments (Fig. 6B). Although PadR forms dimers and oligomers at very low concentrations of glutardhedyde, Usp1 did not, despite its amino acid sequence showing a putative pattern of dimerization (Fig. 1) and despite the high concentrations of glutaraldehyde tested. EMSA results indicate that PadR was able to bind specifically to the padA promoter and, depending on its concentration, to form one or two complexes with the padA promoter probe (Fig. 6C). This binding was specific, as demonstrated by the disappearance of the shift when PadR was preincubated in excess of unlabeled specific padA promoter and by the conservation of the shift in the presence of high concentrations of the unspecific competitor poly(dI-dC) (Fig. 6D). Preincubation of PadR with 10 mM concentration of p-coumaric, ferulic, and o-coumaric acid was unable to abolish the probe shift (Fig. 6E).
FIG. 6.
EMSA analyses of PadR and Usp1 with the padA promoter. (A) SDS-PAGE of proteins extracts containing purified PadR and overexpressed Usp1 used in the EMSA. M, molecular mass standard; R, 2.5 μg of purified PadR-His6; U, 2 μg of protein extract from E. coli pEU in which Usp1 represents ca. 90% of the total protein in the extract. (B) SDS-PAGE of PadR and Usp1 preincubated 10 min with increasing concentrations of glutaraldehyde. (C) EMSA of increasing concentrations from 2 to 20 nM PadR (R) with the padA promoter probe. P, padA promoter DNA probe without PadR. C1 and C2 indicate putative complexes formed between the DNA probe and PadR. (D) EMSA with probe and 5 nM PadR (R5) with addition of a 50-fold (SC50) or a 200-fold (SC200) excess of unlabeled padA promoter fragment as a specific competitor or a 1,000-fold (dIdC1000) excess of competitor poly(dI-dC). (E) EMSA with probe and 5 nM PadR (R5) preincubated for 10 min with 10 mM p-coumaric (Pc), ferulic (Fe), and o-coumaric (Oc) acid. (F) Same as panel E, but without MgCl2 in the binding buffer. (G) EMSA with probe and 5 nM PadR without (R5) and with (R5 pE) total protein extract from E. coli pET28a+ at a protein concentration equivalent to 10 nM Usp1. pE corresponds to the EMSA with probe and the extract of E. coli pET28a+ without cloned gene. (H) EMSA with probe and 5 (U5) or 20 (U20) nM Usp1 extract. R5, the EMSA with probe and 5 nM PadR was used as the positive binding control. (I) EMSA with probe and 5 nM (R5) and 20 nM (R20) PadR, followed by EMSA with probe and 5 nM (R5) PadR, in which PadR was preincubated before the binding reaction for 10 min with increasing concentrations of Usp1 at 1, 2, 5, 10, and 20 nM. (J) Nondenaturing PAGE of PadR and Usp1 produced from recombinant E. coli strains pER and pEU, respectively. These extracts were similar to extracts R and U analyzed in SDS-PAGE in the panel A. R, 3 μg of PadR; U, 4 μg of pEU extract. The lanes correspond to mixes of 4 μg of PadR incubated for 10 min with increasing amounts of pEU extract (2, 4, and 8 μg) in 20 μl of binding buffer. Binding reactions for panels C, D, E, G, H, and I were carried out in standard conditions (with 2.5 mM MgCl2).
Taking into account the results of our recent work on the PASR in B. subtilis (33), which suggested a possible interference of MgCl2 in EMSA, the EMSA was performed in the same conditions as those shown in Fig. 6E but without MgCl2 in the binding buffer (Fig. 6F). In contrast to the results obtained with B. subtilis, the addition of phenolic acid did not abolish the binding of PadR to the padA promoter. To investigate whether Usp1 binds to the padA promoter, serving as a competitor for this promoter or modifying the affinity of PadR to the padA promoter, different EMSAs with the padA promoter were carried out (Fig. 6G and H). A concentrate of crude protein extract from E. coli carrying the vector pET28a+ without the usp1 gene was unable to abolish the binding of the padA promoter with PadR (Fig. 6G). Usp1 was also unable to bind to the padA promoter (Fig. 6H), demonstrating that Usp1 had no affinity for the padA promoter and is not a competitor of padR for the padA promoter.
Since Usp1 inactivates PadR (Fig. 4), EMSAs were carried out with PadR preincubated or not with Usp1 extract, in the same range of concentrations (Fig. 6I). The results indicate that Usp1 abolishes the binding of PadR to the padA promoter, and they support the inactivation of PadR by Usp1 in E. coli pEDRU (Fig. 4), despite high levels of PadR in the bacteria (Fig. 5D). Since PadR and Usp1 might form a coprecipitate, a nonspecific interaction that could account for the inability of PadR to bind to the padA promoter, individual and mixed protein extracts of the two proteins were resolved in nondenaturing PAGE (Fig. 6J) without heating the sample before loading. Although the two proteins have similar migration rates in SDS-PAGE (Fig. 6A), they display distinct migration rates in their native form in a PAGE (Fig. 6J, lanes R and U). Therefore, mixed extracts from purified PadR and increasing concentrations of Usp1 extract were incubated and resolved by PAGE (Fig. 6J, lanes U2R to U8R). In the mixed extracts, each protein conserved perfectly the same migration rate and no other protein band corresponding to a putative PadR-Usp1 coprecipitate was detected.
DISCUSSION
The aim of this study was to elucidate genetic and biochemical mechanisms of the phenolic acid stress response in L. plantarum, a specific and strong stress response induced by some of these chemicals. Sequence and transcriptional analysis of the L. plantarum padA locus indicates that padR, which encodes the negative repressor of padA, is divergently oriented from padA. PadR also forms a bicistronic operon with a gene of unknown function named usp1, since its deduced product shares significant identity with proteins belonging to the USP family. However, the phylogenetic tree (Fig. 2) clearly indicates that Usp1 forms a subfamily with another putative USP of L. plantarum (LP-2993) and two putative USPs from P. pentosaceus. The expression of the padR-usp1 operon specifically induced by phenolic acids is autoregulated by PadR itself (24). Taking together the fact that usp1 is overexpressed during the PASR and the fact that expression of USPs is induced by a large variety of stresses such as the entry in the stationary growth phase and various situations of starvation of bacteria (29, 34), we hypothesized that Usp1 may be involved in the PASR. To address this, a Δusp1 mutant was constructed and tested for its ability to induce the p-coumaric acid-dependent PAD activity. Although the Δusp1 mutant conserved the wild-type phenotype, the usp1 expression was specifically induced by p-coumaric acid, whereas the three usp1 highest similar paralogous genes of L. plantarum were not. These results prompted us to study the possible interaction between PadR and Usp1 in a heterologous system devoid of the PASR.
Coexpression of padR and usp1 demonstrated that Usp1 produced in E. coli was able to inactivate PadR, while the addition of p-coumaric acid to a recombinant E. coli pEDR expressing PadR could not, as it was also observed in recombinant E. coli pJPADP1 expressing the padAR operon from P. pentosaceus (4). All of these results provide evidence that PadR is not inactivated directly by p-coumaric acid or by one of the six putative USPs found in the E. coli genome (28) and independently of the stage of growth (data not shown). This is in agreement with the phylogenetic sequence analysis, which clusters Usp1 in a distinct family from the E. coli USPs (Fig. 2).
The capacity of Usp1 to inactivate PadR was confirmed by EMSA with PadR preincubated or not with Usp1. It is to our knowledge the first time that the capacity of a USP to inactivate a transcriptional regulator is demonstrated, and it is possible that Usp1 modulates other regulators in the same fashion. USPs could act as coordinators of regulator status during stresses. Although PadR and Usp1 possess conserved and characterized dimerization domains (16, 27) (Fig. 1), Usp1 does not seem to be able to form homodimers nor heterodimers with PadR. According to the putative ATP binding domains of PadR (16) and Usp1 (Fig. 1) (27), ATP exchange between Usp1 and PadR could be suspected to be involved in the inactivation of PadR. Usp1 should not act directly with PadR in living L. plantarum cells, but could act as an ATP donor. It was found in E. coli that the addition of ATP to GroEL complexes releases the UspG form, and conversely, that the presence of ATP blocks the interaction of UspG with GroEL (5). Moreover, it was demonstrated in E. coli that the MJ0577 protein, a Usp1 paralogue able to bind ATP, requires an additional cellular factor for ATP hydrolysis. Phosphorylation studies of PadR and Usp1, and the possible transfer of phosphate from Usp1 to PadR during the PASR, will be studied soon. Such a mechanism might depend on the environment of the regulators. Hence, to address this, it is necessary to overexpress and purify these two proteins from the native host L. plantarum, as was done for LmrR (26). Functional analysis of the proteins expressed in E. coli versus those expressed in its native host L. plantarum will be undertaken.
In mammals, phenolic acids, particularly ferulic and caffeic acids, are diet compounds strongly associated with a reduced risk of developing chronic diseases (25). This effect is in part attributed to their antioxidant properties. However, studies have shown that caffeic acid can also trigger DNA degradation in the presence of cupper ions due to its pro-oxidant activity (35, 37). Caffeic acid, a PASR inducer like ferulic and p-coumaric acids, has a strong pro-oxidant effect due to the presence of an ortho-dihydroxyl group that chelates O2 with Cu2+ ions and generates reactive oxygen species, which are responsible for the DNA damage. It is important to note that in E. coli, UspA is required for resistance to DNA-damaging agents (11, 17). Thus, we could hypothesize that Usp1, a UspA family protein, might also be involved in resistance to DNA-damaging agents in L. plantarum, and it may explain the advantage for this bacterium to have its expression induced by phenolic acids, potentially DNA-damaging agents. Nevertheless, this hypothesis does not exclude that Usp1 might be directly involved in the PASR. The lack of an evident phenotype of the usp1 mutant in the PASR could be explained by the presence of another protein with functional redundancy expressed when usp1 is knocked out. We provided evidence for this kind of relay in a recent analysis of the B. subtilis response to salicylic acid, another phenolic acid (13). We showed that the absence of sensitivity to salicylic acid of a mutant in the bsdBCD operon defective in the BsdB, C, and D enzymes involved in the decarboxylation of phenylacrylic and hydroxybenzoic acids was taken over by the expression of the padC gene involved in the PASR. In contrast to the simple mutant bsdBCD, the double mutant bsdBCD-padC was sensitive to this phenolic acid. Recent analyses of USPs function in several groups of microorganisms highlight the complexity of the mechanism of action of proteins suspected to be components of the stress response network in cells, but their role remains most often enigmatic (22). Although we provide evidence that usp1 is involved in the global acid stress response of cells entering into the stationary phase, a comparison of transcriptomic and proteomic data from the usp1 mutant and padR mutants versus the wild-type strain will be undertaken to obtain an overview of the Usp1 and PadR roles in the behavior of L. plantarum.
Acknowledgments
J.G. was supported by a Ph.D. grant from INRA and the Conseil Régional de Bourgogne. H.S. and N.P.T. were supported, respectively, by grants from the French Ministère de l'Education Nationale, de la Recherche et de la Technologie and from the Ambassade de France au Vietnam.
We thank Christine Rojas for her laboratory work. We are very grateful to Philippe Sansonetti and Wilmara Salgado, Institut Pasteur, Paris, France, for helpful discussions and revision of the manuscript.
Footnotes
Published ahead of print on 19 June 2009.
REFERENCES
- 1.Aukrust, T., and I. F. Nes. 1988. Transformation of Lactobacillus plantarum with the plasmid pTV1 by electroporation. FEMS Microbiol. Lett. 52:127-132. [Google Scholar]
- 2.Axelsson, L. 1996. Proceedings of the Fifth Symposium on Lactic Acid Bacteria: genetics, metabolism, and applications, Veldhoven, The Netherlands. Antonie van Leeuwenhoek 70(2-4):97-358. [PubMed] [Google Scholar]
- 3.Barthelmebs, L., C. Diviès, and J.-F. Cavin. 2000. Knockout of the p-coumarate decarboxylase gene from Lactobacillus plantarum reveals the existence of two other inducible enzymatic activities involved in phenolic acid metabolism. Appl. Environ. Microbiol. 66:3368-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthelmebs, L., B. Lecomte, C. Diviès, and J.-F. Cavin. 2000. Inducible metabolism of phenolic acids in Pediococcus pentosaceus is encoded by an autoregulated operon which involves a new class of negative transcriptional regulator. J. Bacteriol. 182:6724-6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bochkareva, E. S., A. S. Girshovich, and E. Bibi. 2002. Identification and characterization of the Escherichia coli stress protein UP12, a putative in vivo substrate of GroEL. Eur. J. Biochem. 269:3032-3040. [DOI] [PubMed] [Google Scholar]
- 6.Cavin, J.-F., L. Barthelmebs, and C. Diviès. 1997. Molecular characterization of an inducible p-coumaric acid decarboxylase from Lactobacillus plantarum: gene cloning, transcriptional analysis, overexpression in Escherichia coli, purification, and characterization. Appl. Environ. Microbiol. 63:1939-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, S. N., A. C. Chang, and L. Hsu. 1972. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc. Natl. Acad. Sci. USA 69:2110-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Man, P. J., M. Rogosa, and M. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 10.Derré, I., G. Rapoport, and T. Msadek. 1999. CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in gram-positive bacteria. Mol. Microbiol. 31:117-131. [DOI] [PubMed] [Google Scholar]
- 11.Diez, A., N. Gustavsson, and T. Nyström. 2000. The universal stress protein A of Escherichia coli is required for resistance to DNA damaging agents and is regulated by a RecA/FtsK-dependent regulatory pathway. Mol. Microbiol. 36:1494-1503. [DOI] [PubMed] [Google Scholar]
- 12.Dower, W. J., J. F. Miller, and C. W. Ragsdale. 1988. High-efficiency transformation of Escherichia coli by high-voltage electroporation. Nucleic Acids Res. 16:6127-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duy, N. V., U. Mader, N. P. Tran, J.-F. Cavin, T. Tam le, D. Albrecht, M. Hecker, and H. Antelmann. 2007. The proteome and transcriptome analysis of Bacillus subtilis in response to salicylic acid. Proteomics 7:698-710. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson, L. R., I. F. Lim, A. E. Pearson, J. Ralph, and P. J. Harris. 2003. Bacterial antimutagenesis by hydroxycinnamic acids from plant cell walls. Mutat. Res. 542:49-58. [DOI] [PubMed] [Google Scholar]
- 15.Gibson, T. J. 1984. Studies on the Epstein-Barr virus genome. Ph.D. thesis. Cambridge University, Cambridge, United Kingdom.
- 16.Gury, J., L. Barthelmebs, N. P. Tran, C. Diviès, and J.-F. Cavin. 2004. Cloning, deletion, and characterization of PadR, the transcriptional repressor of the phenolic acid decarboxylase-encoding padA gene of Lactobacillus plantarum. Appl. Environ. Microbiol. 70:2146-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustavsson, N., A. Diez, and T. Nystrom. 2002. The universal stress protein paralogues of Escherichia coli are co-ordinately regulated and co-operate in the defense against DNA damage. Mol. Microbiol. 43:107-117. [DOI] [PubMed] [Google Scholar]
- 18.Hols, P., T. Ferain, D. Garmyn, N. Bernard, and J. Delcour. 1994. Use of homologous expression-secretion signals and vector-free stable chromosomal integration in engineering of Lactobacillus plantarum for alpha-amylase and levanase expression. Appl. Environ. Microbiol. 60:1401-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleerebezem, M., J. Boekhorst, R. van Kranenburg, D. Molenaar, O. P. Kuipers, R. Leer, R. Tarchini, S. A. Peters, H. M. Sandbrink, M. W. Fiers, W. Stiekema, R. M. Lankhorst, P. A. Bron, S. M. Hoffer, M. N. Groot, R. Kerkhoven, M. de Vries, B. Ursing, W. M. de Vos, and R. J. Siezen. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 100:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovacikova, G., W. Lin, and K. Skorupski. 2005. Dual regulation of genes involved in acetoin biosynthesis and motility/biofilm formation by the virulence activator AphA and the acetate-responsive LysR-type regulator AlsR in Vibrio cholerae. Mol. Microbiol. 57:420-433. [DOI] [PubMed] [Google Scholar]
- 21.Kovacikova, G., W. Lin, and K. Skorupski. 2003. The virulence activator AphA links quorum sensing to pathogenesis and physiology in Vibrio cholerae by repressing the expression of a penicillin amidase gene on the small chromosome. J. Bacteriol. 185:4825-4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kvint, K., L. Nachin, A. Diez, and T. Nyström. 2003. The bacterial universal stress protein: function and regulation. Curr. Opin. Microbiol. 6:140-145. [DOI] [PubMed] [Google Scholar]
- 22a.Larkin, M. A., G. Blackshields, N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm, R. Lopez, J. D. Thompson, T. J. Gibson, and D. G. Higgins. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947-2948. [DOI] [PubMed] [Google Scholar]
- 23.Li, Y., Q. Peng, D. Selimi, Q. Wang, A. O. Charkowski, X. Chen, and C. H. Yang. 2009. The plant phenolic compound p-coumaric acid represses gene expression in the Dickeya dadantii type III secretion system. Appl. Environ. Microbiol. 75:1223-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Licandro-Seraut, H., J. Gury, N. P. Tran, L. Barthelmebs, and J.-F. Cavin. 2008. Kinetics and intensity of the expression of genes involved in the stress response tightly induced by phenolic acids in Lactobacillus plantarum. J. Mol. Microbiol. Biotechnol. 14:41-47. [DOI] [PubMed] [Google Scholar]
- 25.Liu, R. H. 2004. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J. Nutr. 134:3479S-3485S. [DOI] [PubMed] [Google Scholar]
- 26.Madoori, P. K., H. Agustiandari, A. J. Driessen, and A. M. Thunnissen. 2009. Structure of the transcriptional regulator LmrR and its mechanism of multidrug recognition. EMBO J. 28:156-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nachin, L., L. Brive, K. C. Persson, P. Svensson, and T. Nyström. 2008. Heterodimer formation within universal stress protein classes revealed by an in silico and experimental approach. J. Mol. Biol. 380:340-350. [DOI] [PubMed] [Google Scholar]
- 28.Nachin, L., U. Nannmark, and T. Nyström. 2005. Differential roles of the universal stress proteins of Escherichia coli in oxidative stress resistance, adhesion, and mobility. J. Bacteriol. 187:6265-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyström, T., and F. C. Neidhardt. 1992. Cloning, mapping, and nucleotide sequencing of a gene encoding a universal stress protein in Escherichia coli. Mol. Microbiol. 6:3187-3198. [DOI] [PubMed] [Google Scholar]
- 30.Posno, M., R. J. Leer, N. Van Luik, M. J. F. Van Giezen, P. T. H. M. Heuvelmans, B. C. Lockman, and P. H. Pouwels. 1991. Incompatibility of Lactobacillus vectors with replicons derived from small cryptic Lactobacillus plasmids and segregational instability of the introduced vectors. Appl. Environ. Microbiol. 57:1822-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 32.Scalbert, A., and G. Williamson. 2000. Dietary intake and bioavailability of polyphenols. J. Nutr. 130:2073S-2085S. [DOI] [PubMed] [Google Scholar]
- 33.Tran, N. P., J. Gury, V. Dartois, T. K. Nguyen, H. Seraut, L. Barthelmebs, P. Gervais, and J.-F. Cavin. 2008. Phenolic acid-mediated regulation of the padC gene, encoding the phenolic acid decarboxylase of Bacillus subtilis. J. Bacteriol. 190:3213-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VanBogelen, R. A., M. E. Hutton, and F. C. Neidhardt. 1990. Gene-protein database of Escherichia coli K-12, edition 3. Electrophoresis 11:1131-1166. [DOI] [PubMed] [Google Scholar]
- 35.Yamanaka, N., O. Oda, and S. Nagao. 1997. Prooxidant activity of caffeic acid, dietary non-flavonoid phenolic acid, on Cu2+-induced low density lipoprotein oxidation. FEBS Lett. 405:186-190. [DOI] [PubMed] [Google Scholar]
- 36.Zarembinski, T. I., L. W. Hung, H. J. Mueller-Dieckmann, K. K. Kim, H. Yokota, R. Kim, and S. H. Kim. 1998. Structure-based assignment of the biochemical function of a hypothetical protein: a test case of structural genomics. Proc. Natl. Acad. Sci. USA 95:15189-15193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng, L. F., F. Dai, B. Zhou, L. Yang, and Z. L. Liu. 2008. Prooxidant activity of hydroxycinnamic acids on DNA damage in the presence of Cu(II) ions: mechanism and structure-activity relationship. Food Chem. Toxicol. 46:149-156. [DOI] [PubMed] [Google Scholar]