Abstract
Begomovirus-DNA-β disease complexes induce different symptom phenotypes in their hosts. To investigate the genetic determinants of the phenotypic differences, Nicotiana spp. and tomato plants were inoculated with infectious clones of Tobacco curly shoot virus (TbCSV)/TbCSV DNA-β (TbCSB) and Tomato yellow leaf curl China virus (TYLCCNV)/TYLCCNV DNA-β (TYLCCNB) pseudorecombinants and showed that TYLCCNB induced characteristic vein-thickening and enation symptoms, while TbCSB only slightly exacerbated the leaf-curling symptoms, regardless of the helper virus being used. The roles of DNA-β-encoded βC1 and a 430-nucleotide fragment containing the A-rich region and the putative βC1 promoter region of the βC1 gene (referred to as AP) in symptom development were further investigated by constructing hybrid satellites in which the βC1 coding region or AP was exchanged between the two satellite molecules. A TYLCCNB hybrid with TbCSB βC1 lost the ability to elicit the vein-thickening and enation phenotypes. TbCSB hybrids containing the TYLCCNB βC1 or AP fragment failed to induce the characteristic vein thickening and enations. A TYLCCNB hybrid having the TbCSB AP fragment produced the enations, but the number of enations was less and their sizes were reduced. Differently from the phloem-specific pattern of the TYLCCNB promoter, a full-length fragment upstream of the TbCSB βC1 gene confers a constitutive β-glucuronidase expression pattern in transgenic tobacco plants. The above results indicate that the DNA-β-encoded βC1 protein is the symptom determinant, but the promoter of the βC1 gene has influence on symptom production.
Geminiviruses are small plant viruses with circular single-stranded DNA (ssDNA) genomes that are encapsidated in unique twinned (geminate) particles. Members of the genus Begomovirus are transmitted by whiteflies (Bemisia tabaci) and infect dicotyledonous plants (42). Begomoviruses have either one or two circular ssDNA genomic components (DNA-A and DNA-B). The DNA-A component is capable of autonomous replication and encapsidation, whereas the DNA-B component encodes two proteins (BC1 and BV1) involved in movement (14). Recently, some monopartite begomoviruses have been found in association with a novel satellite DNA molecule, referred to as DNA-β and now known as a betasatellite (2, 5, 20, 22, 38, 45). DNA-β is approximately half the size of the viral genomic DNA, and apart from a nonanucleotide sequence (TAATATTAC), it has little sequence identity with viral genomic DNA. DNA-β depends on the helper virus for replication and encapsidation and, in turn, is required for the induction of bona fide disease symptoms. DNA-β bears a βC1 open reading frame (ORF) on the complementary-sense strand, which is conserved among distinct betasatellites in terms of position and size. Mutational analyses and constitutive expression have shown that βC1 is a strong pathogenicity/symptom determinant (7, 34, 39).
Begomovirus-DNA-β disease complexes are associated with a wide range of plant species and induce different sets of symptom phenotypes in their natural hosts (25). However, the contributions of the helper virus and the satellite molecule to symptom development are not clear. Tomato yellow leaf curl China virus (TYLCCNV) and Tobacco curly shoot virus (TbCSV) are monopartite begomoviruses associated with DNA-β, but they differ in the symptom phenotypes induced in Nicotiana spp. and Solanum lycopersicum (7, 22). In the present work, we report that the symptom differences between TYLCCNV/TYLCCNV DNA-β (TYLCCNB) and TbCSV/TbCSV DNA-β (TbCSB) are determined by DNA-β and the DNA-β-encoded βC1 protein is the symptom determinant, but the promoter of the βC1 gene has influence on symptom production.
MATERIALS AND METHODS
Cloning of virus constructs.
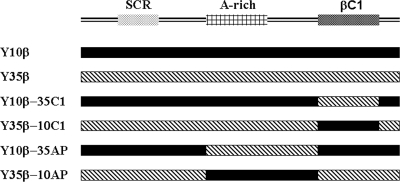
The construction of infectious clones of TYLCCNV isolate Y10 (pBinPLUS-Y10-1.7A), TYLCCNB (pBinPLUS-Y10-1.7β), TbCSV isolate Y35 (pBinPLUS-Y35-1.7A), and TbCSB (pBinPLUS-Y35-1.7β), hereafter referred to as Y10, Y10β, Y35, and Y35β, respectively, has previously been described (22, 45). To construct plasmids containing hybrid DNA-β components, a splicing overlap-extension PCR (SOE-PCR) strategy was employed to precisely exchange the βC1 ORF or the 430-nucleotide (nt) fragment upstream of the βC1 ORF between Y10β and Y35β. The full-length Y10β and Y35β genomes, which had been amplified with the universal abutting primer pair β01/β02 as described previously (4), were used as templates for SOE-PCR. For example, to obtain a chimeric satellite containing the TbCSB βC1 gene in the TYLCCNB sequence context (Y10β-35C1), three independent PCRs were conducted using three pairs of primers (Table 1). The primers β01 and 10βdC1R were used to amplify a fragment covering the region from the 5′ terminus of the satellite conserved region (SCR) to the nucleotide immediately before the start codon of the TYLCCNB βC1 gene, while the primers β02 and 10βdC1F were used to amplify a fragment covering the region extending from the nucleotide immediately after the termination codon of the βC1 gene to the 3′-terminal SCR of TYLCCNB. These two PCRs were conducted with the cloned TYLCCNB as a template, and two fragments excluding the βC1 gene were obtained. The 3′ termini of the primers 10βdC1R and 10βdC1F are complementary to the end and the beginning of the TYLCCNB βC1 gene, respectively, while the 5′-terminal overhangs are complementary to the TbCSB sequences flanking the βC1 gene. In a separate reaction, a third PCR product was amplified with the primer pair 35βC1F/35βC1R, which was designed to recover the entire TbCSB βC1 gene with TYLCCNB flanking sequences at both ends. All PCRs were conducted using Pfu DNA polymerase (Promega, Madison, WI) according to standard procedures. The three PCR products were recovered independently, and 0.5 μl of each product was mixed in the standard PCR system. After annealing and extension were complete, the flanked primer pair β01/β02 was added to amplify the full-length hybrid DNA-β component. The overlapping PCR products were inserted into a pGEM-T Easy vector (Promega) to produce clones pGEM-Y10β-35C1 (in which the βC1 gene of TYLCCNB was substituted by the TbCSB βC1 gene), pGEM-Y35β-10C1 (in which the βC1 gene of TbCSB was substituted by the TYLCCNB βC1 gene), pGEM-Y10β-35AP (in which the fragment containing the A-rich region and the 173-nt putative βC1 promoter region [hereafter abbreviated AP] of TYLCCNB was substituted by that of TbCSB), and pGEM-Y35β-10AP (in which the AP sequence of TbCSB was substituted by that of TYLCCNB), respectively (Fig. 1). All clones were sequenced entirely with the automated model 377 DNA sequencing system (Perkin Elmer, Foster City, CA), and sequence analysis confirmed that successful exchanges occurred without mutations introduced by PCR. Dimeric constructs of hybrid DNA-β clones for agroinoculation, pBinPLUS-Y10β-35C1, pBinPLUS-Y35β-10C1, pBinPLUS-Y10β-35AP, and pBinPLUS-Y35β-10AP, were produced using the method described previously (45).
TABLE 1.
Sequences of primers used for the PCRs
| Primer | Sequence (5′-3′)a | Location |
|---|---|---|
| 35βC1F | CGTATATATATGTATTCATACATTAGCTATTG | 208-228 in Y35β |
| 35βC1R | CAAATAAACATGACAATTAAATACAACAAC | 563-548 in Y35β |
| 10βdC1F | GTTGTTGTATTTAATTGTCATGTTTATTTG | 562-575 in Y10β |
| 10βdC1R | CAATAGCTAATGTATGAATACATATATATACG | 217-195 in Y10β |
| 10βC1F | GATTAAAATACGTATTCATACATCTGAA | 206-222 in Y10β |
| 10βC1R | GAACAAATATGACTATCAAATACAACAACA | 561-545 in Y10β |
| 35βdC1F | TGTTGTTGTATTTGATAGTCATATTTGTTC | 564-576 in Y35β |
| 35βdC1R | TTCAGATGTATGAATACGTATTTTAATC | 219-197 in Y35β |
| Y10PAF | TGATTTACTGCACGGTTTTACTGCGCG | 1011-1025 in Y35β |
| Y35PAR | CGCGCAGTAAAACCGTGCAGTAAATCA | 1002-1011 in Y10β |
| Y10PAR | TACTGATTTACCTCATCATGACGATTT | 989-1001 in Y10β |
| Y35PAF | ATCGTCATGATGAGGTAAATCAGTAATT | 1024-1035 in Y35β |
| Y35C1F | ACCGGATCCATGACTATCAAATACAAC | 585-568 in Y35β |
| Y35C1R | CCGTCGACTCATACATTAGCTATTGTC | 212-230 in Y35β |
| pβC1-F | GAAGCTTATACGTATTTTAATCCGTATG | 211-191 in Y35β |
| pβC1-R | GGATCCATTTGTTCTTGTGACCAAAAC | 569-589 in Y35β |
| β01 | GGTACCACTACGCTACGCAGCAGCC | 1279-1306 |
| β02 | GGTACCTACCCTCCCAGGGGTACAC | 1289-1262 |
The sequences in bold are complementary to DNA-β of TYLCCNV isolate Y10, while the sequences in italic are complementary to DNA-β of TbCSV isolate Y35.
FIG. 1.
Schematic representation of the construction of the hybrid satellite. Betasatellite organization is shown as linear DNA in the complementary sense.
The full-length promoter fragment of the βC1 gene of TbCSB was obtained by PCR amplification with pβC1-F and pβC1-R (Table 1) using pBinPLUS-Y35-1.7β as the template. The amplified fragment was cloned into a pGEM-T Easy vector to generate construct pGEM-βC1. The sequence was then digested with HindIII and BamHI. The resulting fragment was inserted into HindIII/BamHI sites within the binary vector pINT121 (23) to produce pβC1, in which the Cauliflower mosaic virus (CaMV) 35S promoter was substituted with the TbCSB βC1 promoter to drive β-glucuronidase (GUS) expression. The plasmid pINT121 was used as a positive control for GUS expression. For the negative control, the gus-nos fragment was cut from the BamHI/EcoRI sites of pINT121 and inserted into the BamHI/EcoRI sites of the pBinPLUS vector to generate pBinGUS. The pINTβ3-containing promoter fragment of the βC1 gene of TYLCCNB was constructed previously (13).
To obtain the TbCSB βC1 gene (357-nt) construct for plant transformation, plasmid pBinPLUS-Y35-1.7β was used as the template for the PCR with primer pair Y35C1F and Y35C1R (Table 1). After digestion with BamHI and SalI, the PCR fragment was cloned between a duplicated CaMV 35S promoter and the nopaline synthase terminator (nos) in the expression vector pBin438 (21) to produce pBin-Y35βC1. These binary vectors carrying DNA-β constructs were introduced into Agrobacterium tumefaciens strain EHA105 by triparental mating (45).
Agroinoculation of plants.
A. tumefaciens cultures harboring dimeric or partial dimeric constructs in binary vectors were grown at 28°C for 48 h. For the coinoculation of helper virus and DNA-β, equal volumes of the separate bacterial cultures were mixed prior to inoculation. Nicotiana benthamiana, Nicotiana tabacum cv. Samsun nn, Nicotiana glutinosa and S. lycopersicum cv. Hongbaoshi plants were agroinoculated by a stem puncture method. Briefly, a 21-gauge needle was used to inject 0.2 ml of bacterial culture into the stems or the petioles of plants at the four- to six-leaf stage. The inoculated plants were grown in an insect-free cabinet with supplementary lighting to give a 16-h day length and were checked daily for the appearance of symptoms.
Analysis of viral and betasatellite DNAs.
Total nucleic acids were isolated from the young leaves of N. benthamiana and N. glutinosa using the cetyltrimethylammonium bromide method (46). Approximately 5.0 μg of total nucleic acids were separated on 1% agarose gels in TBE buffer (90 mM Tris-borate, 2 mM EDTA [pH 8.3]) and then transferred to Hybond-N+ membranes (Amersham Biosciences, Buckinghamshire, England) by capillary blotting. Membranes were hybridized separately to [α-32P]dCTP-labeled or digoxigenin (DIG)-labeled probes. The virus-specific probes were produced by labeling denatured PCR products of the fragments amplified from the cloned full-length genomes of TbCSV and TYLCCNV, respectively, while the DNA-β probe was produced by labeling an equimolar mixture of the SCRs of TYLCCNB and TbCSB. After prehybridization and hybridization were complete, the blots were washed in 0.1× SSC (1× SSC is 0.15 M sodium chloride plus 0.015 M sodium citrate), 0.1% sodium dodecyl sulfate at 50°C for 30 min. Hybridization signals were detected by phosphorimaging using a Typhoon 9200 imager (Amersham Pharmacia). Hybridization was detected with DIG-labeled probes using the DIG DNA labeling and detection kit according to the manufacturer's instructions (Roche Diagnostics, Mannheim, Germany).
Plant transformation.
N. benthamiana and S. lycopersicum leaf explants were cocultured with agrobacterium by the leaf disc infection method (16), and transformants were selected on Murashige and Skoog medium (27) that was supplemented with 100 mg/liter kanamycin and 500 mg/liter carbenicillin. Kanamycin-resistant shootlets were placed on rooting medium, grown to a height of 5 to 6 cm, and transferred to soil.
Histochemical localization of GUS activity.
GUS histochemical staining was performed essentially as described previously (1, 19). GUS activity was localized histochemically using a staining solution containing 1 mM X-Gluc (Sigma, Aldrich, MO), 50 mM sodium phosphate (pH 7.0), 10 mM sodium EDTA (pH 8.0), 0.5 mM potassium ferrocyanide, 0.5 mM potassium ferricyanide, 20% (vol/vol) methanol, and 0.05% Triton X-100. The samples were infiltrated with substrate and incubated at 37°C for 3 to 12 h. The staining buffer was then removed, and the samples were cleared by sequential changes of 30%, 75%, and 95% ethanol. Sectioning and embedding were performed on some samples (12), which were then examined and photographed under a Leica DC300 stereomicroscope (Leica, Mannheim, Germany).
Quantitative GUS assay.
Fluorometric determination of GUS activity was performed as described by Guan and Zhou (13). The mean GUS activity from the 35S promoter of pINT121 was considered 100% and used to standardize the activity of the promoter in pβC1.
RESULTS
Pseudorecombination and symptoms.
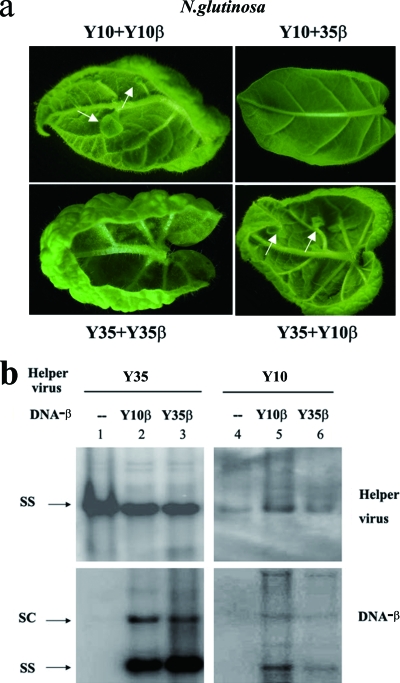
TYLCCNV (Y10) alone could systemically infect its host plants, N. benthamiana, N. glutinosa, N. tabacum cv. Samsun, and tomato plants, but no symptoms or only very mild symptoms were induced (Table 2). However, in the presence of TYLCCNB (Y10β), TYLCCNV induced an array of severe symptoms in the host plants, including leaf curling, vein thickening, leaf distortions on the tops of the leaves, and enations on the undersides of the leaves (Fig. 2a and 4a; Table 2), which sometimes developed into leaflet-like structures on the main veins. In contrast, TbCSV (Y35) alone induced severe leaf-curling symptoms in its hosts, while TbCSB (Y35β) intensified the symptom phenotypes in Nicotiana spp. (Table 2). Symptoms elicited by the coinfection of Y35 and Y35β consisted of pronounced leaf curling (Fig. 2a and 4b; Table 2). To investigate whether the symptom differences were determined by the helper virus or the satellite, pseudorecombinants were produced by the exchange of virus and satellite components. After inoculation with mixed cloned genomic and satellite components Y10 plus 35β or Y35 plus 10β, various host plants became infected (Table 2). N. benthamiana, N. glutinosa, N. tabacum cv. Samsun, and tomato plants infected by Y35 plus Y10β developed severe leaf curling, vein thickening, and enations similar to those induced by Y10 plus Y10β but distinct from the severe leaf-curling symptoms induced by Y35 plus Y35β (Fig. 2a; Table 2). When Nicotiana plants were agroinoculated with Y10 plus Y35β, mild leaf-curling symptoms began to appear at 7 days postinoculation (dpi) (Fig. 2a; Table 2). The mild symptoms coupled with the lower infectivity of this pseudorecombinant (72%, 54%, and 90% compared to 92%, 79%, and 97% for Y10 plus Y10β on N. glutinosa, N. tabacum cv. Samsun, and N. benthamiana, respectively [Table 2]) suggest that TbCSB encodes a weaker pathogenicity factor than TYLCCNB.
TABLE 2.
Infectivity and symptoms induced by TYLCCNV with DNA-β or DNA-β hybrids, TbCSV with DNA-β or DNA-β hybrids, and their pseudorecombinants
| Inoculuma | Plant species | Symptomsb | Infectivityc |
|---|---|---|---|
| Y10 | N. benthamiana | Symptomless | 15/16 (94) |
| N. tabacum cv. Samsun | Symptomless | 10/14 (71) | |
| N. glutinosa | Symptomless | 12/15 (80) | |
| S. lycopersicum | Symptomless | 8/15 (53) | |
| Y10 + Y10β | N. benthamiana | EN, LC, LD, VT | 31/32 (97) |
| N. tabacum cv. Samsun | EN, LC, LD, VT | 22/28 (79) | |
| N. glutinosa | EN, LC, LD, VT | 22/24 (92) | |
| S. lycopersicum | EN, LC, LD, VT | 12/21 (57) | |
| Y10 + Y10β-35C1 | N. benthamiana | LC | 28/30 (93) |
| N. tabacum cv. Samsun | LC | 19/28 (68) | |
| N. glutinosa | LC | 22/25 (88) | |
| S. lycopersicum | LC | 13/20 (65) | |
| Y10 + Y10β-35AP | N. benthamiana | EN, LC, VT | 30/32 (94) |
| N. tabacum cv. Samsun | EN, LC, VT | 18/24 (75) | |
| N. glutinosa | EN, LC, VT | 20/25 (80) | |
| S. lycopersicum | EN, LC, VT | 11/20 (55) | |
| Y10 + Y35β-10AP | N. benthamiana | MLC | 29/32 (90) |
| N. tabacum cv. Samsun | MLC | 10/20 (50) | |
| N. glutinosa | MLC | 13/24 (54) | |
| S. lycopersicum | MLC | 12/20 (60) | |
| Y10 + Y35β | N. benthamiana | MLC | 29/32 (90) |
| N. tabacum cv. Samsun | MLC | 13/24 (54) | |
| N. glutinosa | MLC | 18/25 (72) | |
| S. lycopersicum | MLC | 11/20 (55) | |
| Y35 | N. benthamiana | LC | 20/20 (100) |
| N. tabacum cv. Samsun | LC | 15/16 (94) | |
| N. glutinosa | LC | 18/18 (100) | |
| S. lycopersicum | LC | 19/20 (95) | |
| Y35 + Y10β | N. benthamiana | EN, LC, VT | 31/31 (100) |
| N. tabacum cv. Samsun | EN, LC, VT | 12/12 (100) | |
| N. glutinosa | EN, LC, VT | 24/24 (100) | |
| S. lycopersicum | EN, LC, VT | 19/21 (90) | |
| Y35 + Y35β-10C1 | N. benthamiana | LC | 21/23 (91) |
| N. tabacum cv. Samsun | LC | 32/32 (100) | |
| N. glutinosa | LC | 26/26 (100) | |
| S. lycopersicum | LC | 16/19 (84) | |
| Y35 + Y10β-35AP | N. benthamiana | EN, LC, VT | 31/31 (100) |
| N. tabacum cv. Samsun | EN, LC, VT | 12/12 (100) | |
| N. glutinosa | EN, LC, VT | 24/24 (100) | |
| S. lycopersicum | EN, LC, VT | 15/20 (75) | |
| Y35 + Y35β-10AP | N. benthamiana | LC | 31/32 (97) |
| N. tabacum cv. Samsun | LC | 31/32 (97) | |
| N. glutinosa | LC | 30/32 (94) | |
| S. lycopersicum | LC | 17/20 (85) | |
| Y35 + Y35β | N. benthamiana | LC | 31/32 (97) |
| N. tabacum cv. Samsun | LC | 32/32 (100) | |
| N. glutinosa | LC | 28/28 (100) | |
| S. lycopersicum | LC | 18/20 (90) |
Y10, TYLCCNV; Y10β, TYLCCNB; Y35, TbCSV; Y35β, TbCSB.
EN, enations; LC, leaf curling; LD, leaf distortion; MLC, mild leaf curling; VT, vein thickening.
No. of infected plants/no. of inoculated plants (%) (total of three independent trials).
FIG. 2.
(a) Symptoms induced by TYLCCNV (Y10) and TYLCCNB (Y10β), TbCSV (Y35), and TbCSB (Y35β) and their pseudorecombinants on Nicotiana glutinosa photographed at 60 dpi. Plants were inoculated with Y10 plus Y10β, Y10 plus Y35β, Y35 plus Y10β, and Y35 plus Y35β, respectively. Arrows indicate enations. (b) Southern blot analysis of viral and betasatellite DNAs in inoculated N. glutinosa plants. Total nucleic acids (5 μg) were extracted from the upper leaves of individual plants inoculated with either Y35 alone (lane 1), Y35 plus Y10β (lane 2), Y35 plus Y35β (lane 3), Y10 alone (lane 4), Y10 plus Y10β (lane 5), or Y10 plus Y35β (lane 6). The blots were probed either for the helper virus (top) or for DNA-β (bottom). The positions of single-stranded (SS) and supercoiled (SC) DNA forms are indicated.
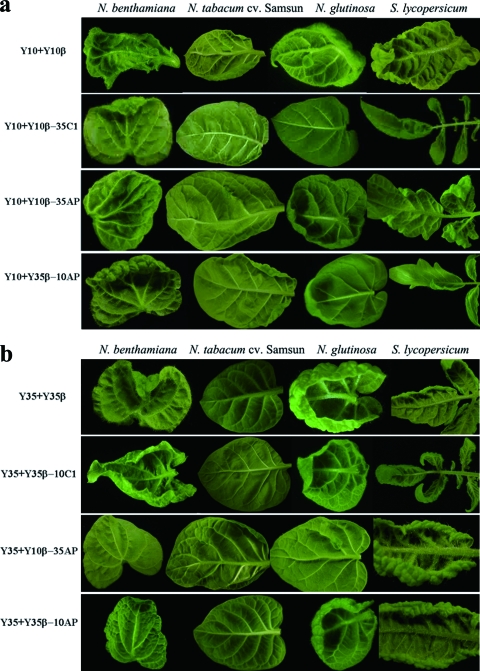
FIG. 4.
Symptoms induced by the coinoculation of chimeric betasatellites with TYLCCNV (Y10) (a) or TbCSV (Y35) (b) on N. benthamiana, N. tabacum cv. Samsun, N. glutinosa, and S. lycopersicum.
Southern blot analyses revealed high levels of satellite DNA-β accumulation in systemically infected tissue of N. glutinosa plants coinoculated with Y35 and either Y10β or Y35β (Fig. 2b, lanes 2 and 3). The levels of Y35 in systemically infected N. glutinosa were largely unaffected by the presence of the satellites (Fig. 2b, lanes 1 to 3). In contrast, Y10β appeared to enhance the accumulation of Y10 in systemically infected N. glutinosa tissues (Fig. 2b, lanes 4 and 5). Plants coinoculated with Y10 plus Y35β accumulated substantially lower levels of both helper virus and satellite than those inoculated with Y10 plus Y10β (Fig. 2b, lanes 5 and 6), which correlated with mild symptoms. In conclusion, the pseudorecombination assay indicated that the enation and vein-thickening phenotypes cosegregated with TYLCCNB (Y10β), while TbCSB (Y35β) mainly intensified leaf-curling symptoms.
Phenotypes of transgenic plants expressing TbCSB βC1.
A previous study showed that transgenic N. benthamiana, N. tabacum, and Arabidopsis spp. plants expressing TYLCCNB βC1 developed morphological abnormalities, such as leaf curling, distortion and small leaf-like enations on the abaxial surface (6, 7, 44). To determine whether the βC1 protein of TbCSB is also responsible for symptom induction, we prepared transgenic N. benthamiana and tomato plants expressing TbCSB βC1 and performed a Northern blot analysis using the TbCSB βC1 gene as a probe (Fig. 3a). Of the rooted plantlets, only 10% of the transgenic N. benthamiana plants were moderately abnormal, including upward leaf curling (Fig. 3b and c). The transgenic tomato plants developed normally and remained symptomless (Fig. 3d and e). Compared with the phenotypes introduced by TbCSB βC1, those introduced by TYLCCNB βC1 were remarkably severe (6, 7, 44) (Fig. 3f). These data indicate that the βC1 protein of TbCSB is inherently distinct from that of TYLCCNB in terms of symptom induction when constitutively expressed in transgenic plants.
FIG. 3.
(a) Northern blot analysis of βC1 mRNA accumulation in transgenic N. benthamiana (lanes 1 and 2) and tomato (lane 3) plants expressing βC1. Lanes 4 and 5 are N. benthamiana and tomato plants, respectively, transformed with the pBin438 vector. Nucleic acid was extracted from the leaves of transgenic plants, and equal amounts of total RNA (15 μg) were loaded into each lane. (b to e) Phenotypes of transgenic N. benthamiana and Solanum lycopersicum expressing the βC1 gene of TbCSB. (b) Example of a moderately abnormal N. benthamiana plant; (c) example of slight upward curling (left) and blistering (right) of N. benthamiana leaves; (d) example of a phenotypically normal S. lycopersicum plant; (e) example of a phenotypically normal S. lycopersicum leaf; (f) example of a severely abnormal S. lycopersicum plant expressing the βC1 gene of TYLCCNB used as a control.
Infectivity and symptoms of hybrid DNA-β.
Since DNA-β-encoded βC1 has been implicated in symptom induction, the symptom differences might be attributed to the βC1 gene alone. Both TYLCCNB and TbCSB encode a 357-nt βC1 gene in their complementary sense strands, and disruption of these ORFs eliminated the satellite-associated phenotypes (7, 22). To further map the symptom determinants, DNA-β hybrids having heterologous βC1 ORFs or fragments containing the A-rich region and the putative promoter region upstream of the translation start site of βC1 (referred to as AP) sequences were generated by SOE-PCR. These constructs, Y10β-35C1, Y35β-10C1, Y10β-35AP, and Y35β-10AP, were individually coinoculated with Y10 or Y35 on N. benthamiana, N. tabacum cv. Samsun, N. glutinosa, and tomato plants. All the DNA-β hybrids systemically infected these four hosts, and most of them induced symptoms different from those elicited by the cognate DNA-β (Table 2; Fig. 4), indicating that all hybrid satellites are viable and that βC1 functions in a heterologous genetic background. The symptoms induced by Y10β-35C1 were Y35β-like and consisted of leaf curling rather than vein thickening and enations. Unexpectedly, Y35β-10C1 also elicited Y35β-like symptoms when coinoculated with Y35, and none of the leaves developed vein thickening and enations during their growth (Fig. 4b). The onset of symptoms (7 to 10 dpi [data not shown]) and the infectivity of both Y10β-35C1 and Y35β-10C1 were similar to those of Y35β (Fig. 4; Table 2). Interestingly, the symptoms induced by Y10β-35AP when coinoculated with either Y10 or Y35 were qualitatively similar to those for the Y10- and Y10β-coinoculated plants, and the number of enations was less and their sizes were reduced (Fig. 4; Table 2). However, vein thickening and enations were not detected in Y35β-10AP-inoculated plants. In conclusion, these results showed that TYLCCNB βC1 is essential but not sufficient to cause the enation phenotype when expressed from the Y35β sequence context and suggest that the upstream sequences, including the putative promoter region of βC1, are also correlated with the symptom severity and the disease phenotype.
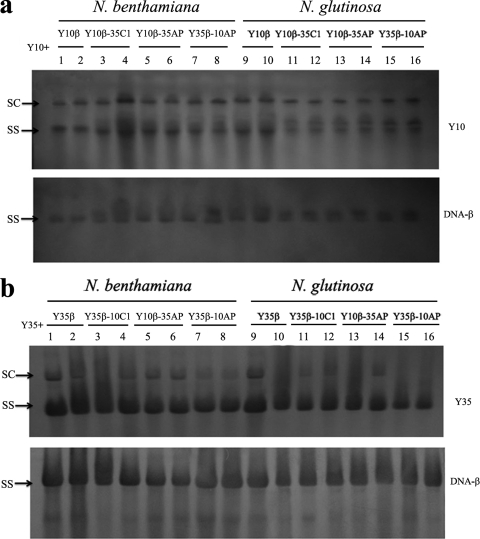
Analysis of viral and betasatellite DNA levels in inoculated plants.
Southern blot analysis of N. benthamiana and N. glutinosa plants agroinoculated with Y10 and Y35 plus wild-type or hybrid satellites revealed predominantly ssDNA with, in some cases, relatively low levels of supercoiled DNA (Fig. 5). The levels of helper virus in systemically infected N. benthamiana and N. glutinosa plants were similar irrespective of which particular DNA-β was present (Fig. 5a and b, top lanes 1 to 16). High levels of Y10β (Fig. 5a, bottom lanes 1, 2, 9, and 10) and the hybrid DNA-βs Y10β-35C1 (Fig. 5a, bottom lanes 3, 4, 11, and 12), Y10β-35AP (Fig. 5a, bottom lanes 5, 6, 13, and 14), and Y35β-10AP (Fig. 5a, bottom lanes 7, 8, 15, and 16) were detected in systemically infected tissues, indicating efficient trans-replication and systemic movement of these satellites by Y10. The same results were noted in tissue systemically infected by Y35β (Fig. 5b, bottom lanes 1, 2, 9, and 10), Y35β-10C1 (Fig. 5b, bottom lanes 3, 4, 11, and 12), Y10β-35AP (Fig. 5b, bottom lanes 5, 6, 13, and 14), and Y35β-10AP (Fig. 5b, bottom lanes 7, 8, 15, and 16) coinoculated with Y35. These data indicate that neither the levels of helper virus nor those of DNA-β correlated with the symptom differences.
FIG. 5.
Southern blot analysis of viral and satellite DNAs extracted from infected N. benthamiana and N. glutinosa plants agroinoculated with TYLCCNV (Y10) together with DNA-β (Y10β) or DNA-β hybrids (a) and TbCSV (Y35) together with DNA-β (Y35β) or DNA-β hybrids (b). Approximately equal amounts (10 μg) of nucleic acids were loaded in each lane. The blots were probed either for Y10 (a, top panel) or Y35 (b, top panel) or for DNA-β (a and b, bottom panels). The positions of single-stranded (SS) and supercoiled (SC) DNA forms are indicated. (a) Lanes 1, 2, 9, and 10, Y10 plus Y10β; lanes 3, 4, 11, and 12, Y10 plus Y10β-35C1; lanes 5, 6, 13, and 14, Y10 plus Y10β-35AP; lanes 7, 8, 15, and 16, Y10 plus Y35β-10AP. (b) Lanes 1, 2, 9, and 10, Y35 plus Y35β; lanes 3, 4, 11, and 12, Y35 plus Y35β-10C1; lanes 5, 6, 13, and 14, Y35 plus Y10β-35AP; lanes 7, 8, 15, and 16, Y35 plus Y35β-10AP.
The putative promoter of TbCSB βC1 confers constitutive GUS expression in transgenic tobacco plants.
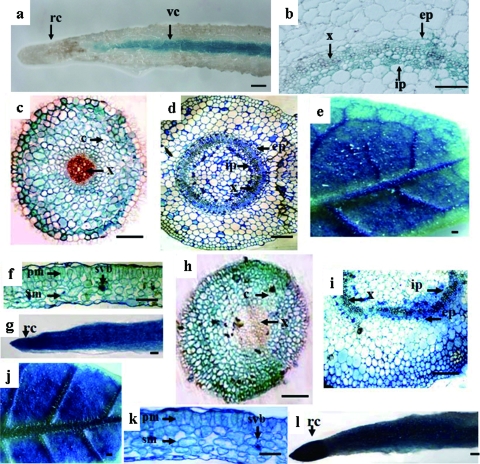
A 955-nt fragment of TYLCCNB, upstream of the translation start site of the βC1 gene, has promoter activity, and GUS expression driven by this fragment is phloem specific (Fig. 6a and b) (13). To determine the expression patterns by the putative promoter from the TbCSB βC1 gene, tobacco plants were transformed with the pβC1, pINT121 containing the CaMV 35S promoter (as a positive control), and pBinGUS (as a negative control) constructs. Eleven independent pβC1 transgenic lines, 10 pINT121 lines, and 3 pBinGUS lines were selected and analyzed using a GUS histochemical staining method. In general, the GUS expression patterns displayed by the pβC1 transgenic line were less intense than those of the pINT121 transgenic line. The blue staining was observed in all tissues except in the primary xylem in the roots (Fig. 6). In transverse sections of the roots prepared from the pβC1 transgenic plants, GUS expression was found to be associated predominantly with the root cortex, and to a lesser extent with the vascular bundles, without apparent staining of the primary xylem (Fig. 6c). Strong GUS staining was observed in both the vascular bundles and mesophyll tissues of leaves (Fig. 6e and f), and in all stem tissue, the vascular phloem was more intensely stained (Fig. 6d). An examination of longitudinal sections from the root tissues revealed that GUS expression driven by pβC1 was located at the root tip as well as the root cap regions (Fig. 6g). Similar expression patterns were also observed in the stem, root, and leaf sections prepared from the pINT121 transgenic line (Fig. 6h to l). As a negative control, no blue staining was detected in pBinGUS transgenic lines (data not shown). These observations indicate that GUS expression driven by pβC1 is constitutive.
FIG. 6.
Histochemical localization of GUS expression patterns in pβC1 and pINT121 transgenic plants. Sections shown in panels a and b were from pINTβ3 transgenic plants showing TYLCCNV satellite promoter expression patterns. Sections shown in panels c, d, e, f, and g were from pβC1 transgenic plants. Sections shown in panels h, i, j, k, and l were from pINT121 transgenic plants. (a) Longitudinal root section; (b) transverse stem section; (c and h) transverse root section; (d and i) transverse stem section; (e and j) underside of leaves; (f and k) transverse leaf section; (g and l) longitudinal root section. c, cortex; ep, external phloem; ip, internal phloem; pm, palisade mesophyll; rc, root cap; sm, spongy mesophyll; svb, secondary vascular bundle; vc, vascular cylinder; x, xylem. Bars = 20 μm.
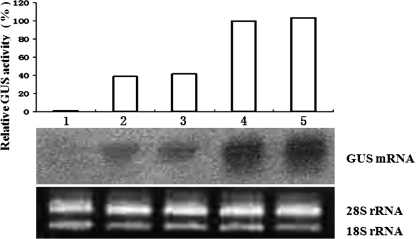
The promoter activity of the GUS fusions was also evaluated in stably transformed kanamycin-resistant plants by fluorometric assays. The results of these assays revealed that GUS activity driven by pβC1 was about 40% of the activity from the CaMV 35S promoter (Fig. 7). Total RNA isolated from the leaves of transgenic tobacco plants was subjected to Northern blot analysis, and the results obtained correlated well with the corresponding GUS fluorometric assays (Fig. 7) in that high levels of GUS activity corresponded to higher accumulation of the GUS transcripts in pINT121 transgenic plants.
FIG. 7.
Relative GUS activity and GUS mRNA accumulation in pβC1 and pINT121 transgenic tobacco plants. The mean GUS activity from the 35S promoter of pINT121 was considered 100% and used to standardize the activity from the promoter in pβC1. Nucleic acid was extracted from the leaves of transgenic plants and analyzed by Northern blotting. Equal amounts of total RNA (15 μg) from each transgenic line were loaded into each lane for the assay, and the blot was hybridized with a probe specific for the GUS gene. Lane 1, untransformed tobacco plant; lanes 2 and 3, plants transformed with pβC1; lanes 4 and 5, plants transformed with pINT121.
DISCUSSION
Bipartite and monopartite begomoviruses are often associated with leaf curling, mosaic, or yellow mosaic symptoms on a variety of dicotyledonous plants. However, the recently identified begomovirus-betasatellite disease complexes commonly induce characteristic phenotypes in their natural hosts, including vein yellowing, leaf curling, vein thickening, and enations (5, 20, 22, 38, 45). Most of the begomoviruses that associate with betasatellites are able to infect the hosts from which they were isolated but require the betasatellite to induce typical symptoms. In an attempt to understand what components of the viral or betasatellite genome are required for the induction of distinct symptom phenotypes, the pseudorecombinants between TYLCCNV/TYLCCNB and TbCSV/TbCSB were inoculated in plants and the determinants of symptom development were localized on the betasatellite. Viable pseudorecombinants have been reported previously between closely related strains or species of bipartite geminiviruses derived from the same geographic region by the reassortment of DNA-A and DNA-B genomic components, such as Tomato golden mosaic virus and Bean golden mosaic virus (31). Pseudorecombination studies, together with the construction of hybrid viruses, have implicated both protein-encoding regions and noncoding regions of DNA-A and DNA-B as genetic determinants of multiple viral biological properties. These properties include host adaptation (11, 30, 31, 41), tissue tropism (26, 33), host range (15, 17), symptomatology (9, 40), and an avirulence determinant conditioning a hypersensitive reaction (10). In contrast to the highly specific recognition of the bipartite geminiviral origin of DNA replication by viral replication-associated proteins (Rep), the betasatellites appear to be able to use the Rep proteins from a diverse range of monopartite begomoviruses (3, 24).
Comparisons of the sequences of betasatellites associated with distinct begomoviruses have resulted in the identification of several common features. These include a highly conserved noncoding region (SCR), which includes the stem-loop structure and the universal nonanucleotide TAATATTAC in the loop; a conserved βC1 ORF in the complementary-sense strand; and an adenosine-rich (A-rich) region varying between 160 and 280 bases that separates the βC1 ORF and the SCR (3, 25). In both Ageratum yellow vein virus (AYVV)- and Cotton leaf curl Multan virus (CLCuMV)-infected plants, naturally occurring recombinants of approximately the same size as DNA-β have been characterized. These recombinants, typified by recDNA-Aβ17, contain sequences derived from the intergenic region of the helper virus, the βC1-encoding region, and flanking sequences derived from DNA-β (5). Saunders et al. (37) demonstrated that Ageratum conyzoides plants coinoculated with AYVV and recDNA-Aβ17 display a yellow-vein phenotype indistinguishable from that associated with DNA-A and DNA-β. Similarly, Tao and Zhou (43) also showed that such recombinants associated with TYLCCNV/DNA-β-infected plants were able to induce typical disease symptoms when coinoculated with TYLCCNV. Since all recombinants lack the SCR, these results indicated that the SCR is not involved in symptom induction.
Mutagenesis of the betasatellite associated with TYLCCNV, AYVV, CLCuMV, and TbCSV has also revealed that βC1 is required for disease symptom induction. In addition, the transgenic expression of βC1 causes disease-like symptoms, indicating that βC1 encodes a pathogenicity determinant (7, 22, 34, 39). The role of the βC1 gene is reminiscent of that of BC1 of bipartite begomoviruses, which encodes a movement protein required for the cell-to-cell movement of DNA-A (28, 36). A recent report indicates that the DNA-β associated with CLCuMV can substitute for the DNA-B of Tomato leaf curl New Delhi virus to permit systemic infection (35). BC1 appears to be a symptom determinant for bipartite geminiviruses, and the transgenic expression of the BC1 gene of Squash leaf curl virus in N. benthamiana (29), Tomato mottle virus in N. tabacum (8), and Bean dwarf mosaic virus in tomato plants (18) induces developmental abnormalities. Sequence analysis shows that TYLCCNB (AJ421621) and TbCSB (AJ411484) share 56% nucleotide sequence identity and that their βC1 proteins share 71% amino acid sequence identity. Hybrid satellites were constructed to precisely shuttle the βC1 ORF between the two satellites. The hybrid satellite Y10β-35C1 containing TbCSB βC1 in TYLCCNB lost the ability to induce vein thickening and enations, indicating that TYLCCNB βC1 plays an important role in the characteristic phenotypes. Corresponding with this observation, significant differences are also evident in the transgenic phenotypes between TYLCCNVB βC1 and TbCSB βC1. Only 10% of the transgenic N. benthamiana plants expressing TbCSB βC1 displays modest leaf curling, and the transgenic tomato plants remained symptomless (Fig. 3). This is consistent with the previous demonstration that TbCSB could intensify the symptoms induced by TbCSV on Nicotiana spp. but not on tomato plants. Surprisingly, the reciprocal hybrid (Y35β-10C1) did not restore the wild-type TYLCCNB phenotype but instead induced symptoms similar to those of TbCSB. These results indicate that TYLCCNB βC1 is essential for vein thickening and enations but not sufficient to cause this phenotype when expressed from a TbCSB background.
The βC1 promoter of TYLCCNB has been demonstrated to confer a phloem-specific expression pattern in transgenic tobacco, and a 173-nt fragment from the 3′ end is sufficient to drive the expression pattern but the A-rich region slightly regulates the promoter activity (13). To further elucidate additional sequences important for the vein-thickening and enation phenotypes, hybrid satellites were constructed by precisely shuttling the A-rich region and a 173- or 200-nt putative promoter fragment (referred to as AP) between the two satellites. The hybrid Y10β-35AP could produce enations, but these enations were smaller and less pronounced than those of wild-type TYLCCNB. The attenuation of enation production can be explained by the differences in the expression patterns of the βC1 promoters. So the promoter of the TbCSB βC1 gene was assumed to confer a different expression pattern from that of TYLCCNB βC1. The results confirm that a full-length fragment upstream of the TbCSB βC1 gene confers a constitutive GUS expression pattern in transgenic tobacco plants. Interestingly, N. benthamiana and N. tabacum plants transformed with a construct containing the βC1 gene of TYLCCNB under the control of the CaMV 35S promoter display interveinal protuberances or small interveinal tissue outgrowths on the undersides of the leaves (7) but no enations. This result can be attributed to the constitutive expression of the CaMV 35S promoter. Although βC1 is the symptom determinant, symptoms also depend upon where the βC1 gene is expressed; thus, the promoter of the βC1 gene has influence on symptom production. Similar results were obtained by Qazi et al. (32), who found that the phloem-specific nature of the βC1 promoter plays a role in tissue specificity for the symptom expression of cotton leaf curl disease.
Now different symptoms induced by TbCSV/TbCSB and TYLCCNV/TYLCCNB can be explained partly. The expression of the βC1 gene of TYLCCNB can induce abnormal cell division (6) and its promoter is phloem specific (13), so TYLCCNV/TYLCCNB induces vein thickening and enations in its infected hosts. For TbCSB, βC1 expression cannot induce abnormal cell division (Fig. 3) and its promoter is constitutive, so TbCSV/TbCSB does not induce vein thickening and enations in its infected hosts.
In conclusion, the symptom differences of TYLCCNV/TYLCCNB and TbCSV/TbCSB are determined by DNA-β and the DNA-β-encoded βC1 protein is the symptom determinant, but the promoter of the βC1 gene has influence on symptom production.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grants 30530520 and 30770092), the National Key Basic Research and Development Program (2006CB101903), and the National Science & Technology Specific Project (2009ZX08009-134B).
Footnotes
Published ahead of print on 19 June 2009.
REFERENCES
- 1.Battraw, M. J., and T. C. Hall. 1990. Histochemical analysis of CaMV 35S promoter-β-glucuronidase gene expression in transgenic rice plants. Plant Mol. Biol. 15:527-538. [DOI] [PubMed] [Google Scholar]
- 2.Briddon, R. W., J. K. Brown, E. Moriones, J. Stanley, M. Zerbini, X. Zhou, and C. M. Fauquet. 2008. Recommendations for the classification and nomenclature of the DNA-β satellites of begomoviruses. Arch. Virol. 153:763-781. [DOI] [PubMed] [Google Scholar]
- 3.Briddon, R. W., S. E. Bull, I. Amin, A. M. Idris, S. Mansoor, I. D. Bedford, and P. Dhawan. 2003. Diversity of DNAβ, a satellite molecule associated with some monopartite begomoviruses. Virology 312:106-121. [DOI] [PubMed] [Google Scholar]
- 4.Briddon, R. W., S. E. Bull, S. Mansoor, I. Amin, and P. G. Markham. 2002. Universal primers for the PCR-mediated amplification of DNAβ—a molecule associated with some monopartite begomoviruses. Mol. Biotechnol. 20:315-318. [DOI] [PubMed] [Google Scholar]
- 5.Briddon, R. W., S. Mansoor, I. D. Bedford, M. S. Pinner, K. Saunders, J. Stanley, Y. Zafar, K. A. Malik, and P. G. Markham. 2001. Identification of DNA components required for induction of cotton leaf curl disease. Virology 285:234-243. [DOI] [PubMed] [Google Scholar]
- 6.Cui, X. F., Y. Q. Li, D. W. Hu, and X. P. Zhou. 2005. Expression of a begomoviral DNAβ gene in transgenic Nicotiana plants induced abnormal cell division. J. Zhejiang Univ. Sci. B 6:83-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui, X. F., X. R. Tao, Y. Xie, C. M. Fauquet, and X. P. Zhou. 2004. A DNAβ associated with Tomato Yellow Leaf Curl China Virus is required for symptom induction. J. Virol. 78:13966-13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan, Y. P., C. A. Powell, D. E. Purcifull, P. Broglio, and E. Hiebert. 1997. Phenotypic variation in transgenic tobacco expressing mutated geminivirus movement/pathogenicity (BC1) proteins. Mol. Plant-Microbe Interact. 10:1065-1074. [DOI] [PubMed] [Google Scholar]
- 9.Garrido-Ramirez, E. R., M. R. Sudarshana, and R. L. Gilbertson. 2000. Bean golden yellow mosaic virus from Chiapas, Mexico: characterization, pseudorecombination with other bean-infecting geminiviruses and germplasm screening. Phytopathology 90:1224-1232. [DOI] [PubMed] [Google Scholar]
- 10.Garrido-Ramirez, E. R., M. R. Sudarshana, W. J. Lucas, and R. L. Gilbertson. 2000. Bean dwarf mosaic virus BV1 protein is a determinant of the hypersensitive response and avirulence in Phaseolus vulgaris. Mol. Plant-Microbe Interact. 13:1184-1194. [DOI] [PubMed] [Google Scholar]
- 11.Gillette, W. K., T. J. Meade, J. L. Jeffrey, and I. T. D. Petty. 1998. Genetic determinants of host-specificity in bipartite geminivirus DNA A components. Virology 251:361-369. [DOI] [PubMed] [Google Scholar]
- 12.Glauert, A. M. (ed.). 1972. Practical methods in electron microscopy. Specimen preparation in materials science, part 1, vol. I. Elsevier, New York, NY.
- 13.Guan, C. P., and X. P. Zhou. 2006. Phloem-specific promoter from a satellite associated with a DNA virus. Virus Res. 115:150-157. [DOI] [PubMed] [Google Scholar]
- 14.Hanley-Bowdoin, L., S. B. Settlage, B. M. Orozco, S. Nagar, and D. Robertson. 2000. Geminiviruses: models for plant DNA replication, transcription, and cell cycle regulation. Crit. Rev. Biochem. Mol. Biol. 35:105-140. [PubMed] [Google Scholar]
- 15.Hill, J. E., J. O. Strandberg, E. Hiebert, and S. G. Lazarowitz. 1998. Asymmetric infectivity of pseudorecombinants of cabbage leaf curl virus and squash leaf curl virus: implications for bipartite geminivirus evolution and movement. Virology 250:283-292. [DOI] [PubMed] [Google Scholar]
- 16.Horsch, R. B., J. E. Fry, N. L. Hoffman, D. Eichholtz, S. G. Rogers, and R. T. Fraley. 1985. A simple and general method for transferring genes into plants. Science 227:1229-1231. [DOI] [PubMed] [Google Scholar]
- 17.Hou, Y. M., E. J. Paplomatas, and R. L. Gilbertson. 1998. Host adaptation and replication properties of two bipartite geminiviruses and their pseudorecombinants. Mol. Plant-Microbe Interact. 11:208-217. [Google Scholar]
- 18.Hou, Y. M., R. Sanders, V. M. Ursin, and R. L. Gilberston. 2000. Transgenic plants expressing geminivirus movement proteins: abnormal phenotypes and delayed infection by tomato mottle virus in transgenic tomatoes expressing the bean dwarf mosaic virus BV1 or BC1 proteins. Mol. Plant-Microbe Interact. 13:297-308. [DOI] [PubMed] [Google Scholar]
- 19.Jefferson, R. A., T. A. Kavanagh, and M. W. Bevan. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6:3901-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jose, J., and R. Usha. 2003. Bhendi yellow vein mosaic disease in India is caused by association of a DNAβ satellite with a begomovirus. Virology 305:310-317. [DOI] [PubMed] [Google Scholar]
- 21.Li, T. Y., Y. C. Tian, and X. F. Qin. 1994. Transgenic tobacco plants with efficient insect resistance. Sci. China B 37:1479-1489. [Google Scholar]
- 22.Li, Z. H., X. P. Zhou, and Y. Xie. 2005. Tobacco curly shoot virus DNAβ is not necessary for infection but may intensify symptoms in a host-dependent manner. Phytopathology 95:902-908. [DOI] [PubMed] [Google Scholar]
- 23.Liu, Z. Z., J. L. Wang, X. Huang, W. H. Xu, Z. M. Liu, and R. X. Fang. 2003. The promoter of a rice glycine-rich protein gene, Osgrp-2, confers vascular-specific expression in transgenic plants. Planta 216:824-833. [DOI] [PubMed] [Google Scholar]
- 24.Mansoor, S., R. W. Briddon, S. E. Bull, I. D. Bedford, A. Bashir, M. Hussain, and M. Saeed. 2003. Cotton leaf curl disease is associated with multiple monopartite begomoviruses supported by single DNAβ. Arch. Virol. 148:1969-1986. [DOI] [PubMed] [Google Scholar]
- 25.Mansoor, S., R. W. Briddon, Y. Zafar, and J. Stanley. 2003. Geminivirus disease complexes: an emerging threat. Trends Plant Sci. 8:128-134. [DOI] [PubMed] [Google Scholar]
- 26.Morra, M. R., and I. T. D. Petty. 2000. Tissue specificity of geminivirus infection is genetically determined. Plant Cell 12:2259-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murashige, T., and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473-497. [Google Scholar]
- 28.Noueiry, A. O., W. J. Lucas, and R. L. Gilbertson. 1994. Two proteins of a plant DNA virus coordinate nuclear and plasmodesmal transport. Cell 76:925-932. [DOI] [PubMed] [Google Scholar]
- 29.Pascal, E., P. E. Goodlove, L. C. Wu, and S. G. Lazarowitz. 1993. Transgenic tobacco plants expressing the geminivirus BL1 protein exhibit symptoms of viral disease. Plant Cell 5:795-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petty, I. T. D., S. C. Carter, M. R. Morra, J. L. Jeffrey, and H. E. Olivey. 2000. Bipartite geminivirus host adaptation determined cooperatively by coding and noncoding sequences of the genome. Virology 277:429-438. [DOI] [PubMed] [Google Scholar]
- 31.Petty, I. T. D., C. G. Miller, T. J. Meadehash, and R. L. Schaffer. 1995. Complementable and noncomplementable host adaptation defects in bipartite geminiviruses. Virology 212:263-267. [DOI] [PubMed] [Google Scholar]
- 32.Qazi, J., I. Amin, S. Mansoor, J. Iqbal, and R. W. Briddon. 2007. Contribution of the satellite encoded gene βC1 to cotton leaf curl disease symptoms. Virus Res. 128:135-139. [DOI] [PubMed] [Google Scholar]
- 33.Qin, Y., and I. T. D. Petty. 2001. Genetic analysis of bipartite geminivirus tissue tropism. Virology 291:311-323. [DOI] [PubMed] [Google Scholar]
- 34.Saeed, M., S. A. A. Behjatnia, S. Mansoor, Y. Zafar, S. Hasnain, and M. A. Rezaian. 2005. A single complementary-sense transcript of a geminiviral DNAβ satellite is determinant of pathogenicity. Mol. Plant-Microbe Interact. 18:7-14. [DOI] [PubMed] [Google Scholar]
- 35.Saeed, M., Y. Zafar, J. W. Randles, and M. A. Rezaian. 2007. A monopartite begomovirus-associated DNAβ satellite substitutes for the DNA B of a bipartite begomovirus to permit systemic infection. J. Gen. Virol. 88:2881-2889. [DOI] [PubMed] [Google Scholar]
- 36.Sanderfoot, A. A., and S. G. Lazarowitz. 1996. Getting it together in plant virus movement: cooperative interactions between bipartite geminivirus movement proteins. Trends Cell Biol. 6:353-358. [DOI] [PubMed] [Google Scholar]
- 37.Saunders, K., I. D. Bedford, and J. Stanley. 2001. Pathogenicity of a natural recombinant associated with ageratum yellow vein disease: implications for geminivirus evolution and disease aetiology. Virology 282:38-47. [DOI] [PubMed] [Google Scholar]
- 38.Saunders, K., I. D. Bedford, R. W. Briddon, P. G. Markham, S. M. Wong, and J. Stanley. 2000. A unique virus complex causes ageratum yellow vein disease. Proc. Natl. Acad. Sci. USA 97:6890-6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saunders, K., A. Norman, S. Gucciardo, and J. Stanley. 2004. The DNAβ satellite component associated with ageratum yellow vein disease encodes an essential pathogenicity protein (βC1). Virology 324:37-47. [DOI] [PubMed] [Google Scholar]
- 40.Saunders, K., C. Wege, K. Veluthambi, H. Jeske, and J. Stanley. 2001. The distinct disease phenotypes of the common and yellow vein strains of tomato golden mosaic virus are determined by nucleotide differences in the 3′-terminal region of the gene encoding the movement protein. J. Gen. Virol. 82:45-51. [DOI] [PubMed] [Google Scholar]
- 41.Schaffer, R. L., C. G. Miller, and I. T. D. Petty. 1995. Virus- and host-specific adaptations in the BL1 and BR1 genes of bipartite geminiviruses. Virology 214:330-338. [DOI] [PubMed] [Google Scholar]
- 42.Stanley, J., D. M. Bisaro, R. W. Briddon, J. K. Brown, C. M. Fauquet, B. D. Harrison, E. P. Rybicki, and D. C. Stenger. 2005. Geminiviridae, p. 301-326. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy. VIIIth report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press, London, United Kingdom.
- 43.Tao, X. R., and X. P. Zhou. 2008. Pathogenicity of a naturally occurring recombinant DNA satellite associated with tomato yellow leaf curl China virus. J. Gen. Virol. 89:306-311. [DOI] [PubMed] [Google Scholar]
- 44.Yang, J. Y., M. Iwasaki, C. Machida, Y. Machida, X. P. Zhou, and N.-H. Chua. 2008. βC1, the pathogenicity factor of TYLCCNV, interacts with AS1 to alter leaf development and suppress selective jasmonic acid responses. Genes Dev. 22:2564-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou, X. P., Y. Xie, X. R. Tao, Z. K. Zhang, Z. H. Li, and C. M. Fauquet. 2003. Characterization of DNAβ associated with begomoviruses in China and evidence for co-evolution with their cognate viral DNA-A. J. Gen. Virol. 84:237-247. [DOI] [PubMed] [Google Scholar]
- 46.Zhou, X. P., Y. Xie, Z. K. Zhang, Y. J. Qi, and J. J. Wu. 2001. Molecular characterization of a novel defective DNA isolated from tobacco tissues infected with tobacco leaf curl virus. Acta Virol. 45:45-50. [PubMed] [Google Scholar]