Abstract
In an effort to characterize important epitopes of Staphylococcus aureus iron-regulated surface determinant B (IsdB), murine IsdB-specific monoclonal antibodies (MAbs) were isolated and characterized. A panel of 12 MAbs was isolated. All 12 MAbs recognized IsdB in enzyme-linked immunosorbent assays and Western blots; 10 recognized native IsdB expressed by S. aureus. The antigen epitope binding of eight of the MAbs was examined further. Three methods were used to assess binding diversity: MAb binding to IsdB muteins, pairwise binding to recombinant IsdB, and pairwise binding to IsdB-expressing bacteria. Data from these analyses indicated that MAbs could be grouped based on distinct or nonoverlapping epitope recognition. Also, MAb binding to recombinant IsdB required a significant portion of intact antigen, implying conformational epitope recognition. Four MAbs with nonoverlapping epitopes were evaluated for in vitro opsonophagocytic killing (OPK) activity and efficacy in murine challenge models. These were isotype switched from immunoglobulin G1 (IgG1) to IgG2b to potentially enhance activity; however, this isotype switch did not appear to enhance functional activity. MAb 2H2 exhibited OPK activity (≥50% killing in the in vitro OPK assay) and was protective in two lethal challenge models and a sublethal indwelling catheter model. MAb 13C7 did not exhibit OPK (<50% killing in the in vitro assay) and was protective in one lethal challenge model. Neither MAb 13G11 nor MAb 1G3 exhibited OPK activity in vitro or was active in a lethal challenge model. The data suggest that several nonoverlapping epitopes are recognized by the IsdB-specific MAbs, but not all of these epitopes induce protective antibodies.
The search for an efficacious vaccine or immunoglobulin (Ig) preparation to prevent invasive disease due to Staphylococcus aureus has proceeded for well over a decade (17, 22, 23, 26). However, with the recent dramatic increase in cases of methicillin-resistant S. aureus (12, 13), this effort has become more urgent. S. aureus has increasingly become a pathogen of great clinical concern over the last 3 decades (7, 12, 13, 16). The importance of S. aureus vaccine development to aid in the treatment of hospitalized individuals, as well as to reduce the economic burden on the health care system, is well established.
Although extensively investigated, native protective immunity against S. aureus is poorly understood. Acute infection with S. aureus does not prevent reinfection with this bacterium (17). Preclinical and clinical data indicate that immunization with intact whole bacteria induces high immune titers to staphylococcus but does not confer protection from S. aureus disease (10, 17). Clearance of S. aureus is thought to be dependent upon antibody and complement-mediated uptake and killing by neutrophils, known as opsonophagocytic killing (OPK) (6, 11, 18, 19, 24, 33). S. aureus is a part of the normal bacterial flora of humans. As such, all individuals have antibodies to S. aureus, perhaps due to repeated subclinical infections or to carriage of the bacteria on mucosal surfaces in the nares, rectum, vagina, etc. Humans, as well as many mammals, have preexisting antibody titers to iron-regulated surface determinant B (IsdB) (15), but it is unknown whether these preexisting titers offer protection. Confirmed protection by natural antibodies to individual S. aureus antigens has been demonstrated for a single antigen, staphylococcal toxic-shock toxin 1 (17). Other antibodies to individual antigens have been proposed to correlate with natural protection, such as an immunodominant ABC transporter described by Burnie and coauthors (3) and antigens described by Clarke and coauthors (4). Several polysaccharide and protein antigens have been tested as vaccine candidates for S. aureus (reviewed in reference 26; 1, 29). Active immunization with these vaccine candidates leads to high titers of IgG which may confer protection from challenge (26). Kuklin et al. demonstrated that immunization with IsdB formulated on amorphous aluminum hydroxyphosphate sulfate adjuvant increased murine antibody titers by up to 20-fold and nonhuman primate titers by fourfold. Importantly, increased antibody titers correlated with enhanced survival in a murine lethal challenge model (15). IsdB is an antigen expressed on the cell surface of S. aureus in environments with limited iron, with a molecular mass of approximately 72 kDa. Its function is to capture and import heme iron from hemoglobin (20). Since little is known about the protective immune response to IsdB, the current study was undertaken to investigate IsdB-specific antibodies which may confer protection.
In an effort to further our understanding of potentially protective IsdB epitopes, IsdB-specific murine monoclonal antibodies (MAbs) were selected and characterized. An understanding of the protective epitopes of IsdB will inform decisions on the type of antibody response necessary for protection from S. aureus challenge. Epitope-specific and protective MAbs are also important as reagents to ensure the maintenance of appropriate structural integrity of IsdB antigen during vaccine formulation. The IsdB MAbs were grouped based on recognition of similar epitope regions. The MAbs fell into three or four groups depending on the method of analysis. Several nonoverlapping epitopes were delineated by these MAbs, and two were important for in vivo protection in murine challenge models.
MATERIALS AND METHODS
Bacteria.
The bacteria used in this investigation were the following: S. aureus Becker (obtained from Chia Lee, University of Arkansas), S. aureus MCL8538 (Merck repository), and S. aureus RN4220 (obtained from Richard Novick, New York University School of Medicine). Bacteria were grown on tryptic soy agar (TSA) or tryptic soy broth (TSB) overnight, pelleted, and stored as frozen 15% glycerol stocks. Alternatively, bacteria were passaged two to three times to stationary phase in low-iron RPMI medium, pelleted, and stored frozen in 15% glycerol. For use in experiments, bacteria were thawed, pelleted, and resuspended in the appropriate buffer or medium.
Recombinant IsdB and IsdB muteins.
Native isdB was cloned with a C-terminal His tag into the Escherichia coli expression vector pET-28a (Novagen). IsdB muteins were prepared by replacing IsdB amino acids with the corresponding amino acids from IsdH, an antigen bearing high sequence homology with IsdB (8, 25). Mutations were introduced cumulatively. The first mutant was used as the parental plasmid for mutant two in the series, the second was used to build the third, and so on. The isdB expression vector was subjected to mutagenesis using Stratagene's QuikChange multisite-directed mutagenesis kit, following the manufacturer's instructions. After amplification, plasmids were digested with DpnI for 1 h at 37°C and then transformed into Stratagene's XL10-Gold competent cells, following the manufacturer's protocol. Muteins were screened by sequencing using ABI's 310 DNA sequencer. Based on the number of nucleotide changes, one or two plasmids from each round of mutagenesis were selected. These plasmids were transformed into the E. coli expression host HMS174(DE3) (Novagen) and expressed following Novagen's instructions. The His-tagged IsdB muteins were purified by Ni-nitrilotriacetic acid agarose (Qiagen) affinity chromatography. One plasmid from each transformation was then used in the next round of mutagenesis.
MAb preparation.
All animal work was performed in accordance with the Merck Research Laboratories Institutional Animal Care and Use committee guidelines. Two separate immunization protocols were performed in an effort to maximally immunize mice for splenocyte harvest and hybridoma preparation. In the first protocol, mice were immunized intramuscularly three times with IsdB and amorphous aluminum hydroxyphosphate sulfate adjuvant and challenged intravenously (i.v.) with S. aureus Becker, as described previously (15). Surviving mice were boosted i.v. with 20 μg/mouse of IsdB prior to splenocyte harvest. In the second protocol, mice were immunized three times with IsdB as in protocol 1. Mice were not challenged in this protocol. The mice were boosted i.v. with 20 μg/mouse of IsdB as in protocol 1. Hybridomas were prepared using established methodology (14). Lymphocytes prepared from spleens were fused with the mouse myeloma partner SP2/0-Ag14 (ATCC 1581) by using polyethylene glycol 1500 (Boehringer Mannheim) at a ratio of 3:1. The fusions were plated into 96-well, flat-bottomed microtiter plates in Dulbecco's modification of Eagle's medium, high glucose, pyruvate (DMEM) containing 20% fetal bovine serum, hypoxanthine (10−4 M), and thymidine (10−5 M). Aminopterin (4 × 10−7 M) was added 24 h later. Supernatants from growing hybridomas were screened using one of two methods, enzyme-linked immunosorbent assay (ELISA) for reactivity to IsdB (protocol 1) or flow cytometry for surface binding to IsdB-expressing S. aureus Becker (protocol 2), as described previously (15). Positive wells were cloned by limiting dilution and retested for ELISA reactivity. MAbs were classified with an antibody isotyping kit (Roche Diagnostics Corporation, Indianapolis, IN).
Monoclonal class switching from IgG1 to IgG2b.
Antibodies were class switched to the IgG2b isotype by selecting for spontaneous shift variants (30). A suitable immunoassay was developed using an IgG2b conjugate, and the cell line was plated at a high density in 96-well plates. Somatic-cell mutations were selected, enriched, and replated at lower densities and, after several rounds of this, ultimately cloned by limiting dilution.
IsdB ELISA.
The IsdB ELISA was performed as previously described (15).
Surface plasmon resonance (SPR) analysis.
MAbs were grouped into sets based on recognition of overlapping or common IsdB epitopes. The evaluation of MAb epitope recognition on recombinant wild-type IsdB was performed using two (pairwise) approaches as described in Methods in the supplemental material.
Flow cytometry.
Prepared glycerol stocks of S. aureus passaged two to three times under iron-starved conditions (in RPMI medium) were used to evaluate MAbs for IsdB binding, as previously described (5, 15).
MAb binding to native surface-expressed IsdB.
MAbs were evaluated pairwise for recognition of IsdB epitopes available on the surface of appropriately cultured bacteria. Purified antibodies were labeled with Alexa Fluor 488 using a MAb-labeling kit (Molecular Probes) according to the manufacturer's instructions. The amount of MAb that would just saturate the surface of bacterial cells grown in RPMI medium was determined for both the labeled and unlabeled MAbs. Each of the MAbs evaluated was used labeled and unlabeled.
The pairwise binding assay was performed by first incubating 5 × 107 CFU with the unlabeled MAb at a concentration that would saturate the surface of the cells (i.e., unlabeled MAb was added in excess). This reaction mixture was incubated at room temperature for 1 h. After this incubation, the reaction mixtures were washed with 1 ml of PAA (phosphate-buffered saline [PBS], 1% bovine serum albumin [wt/vol], 0.1% NaN3 [wt/vol]) and spun at 6,000 rpm for 5 min in a microcentrifuge (Hermle). The supernatant was removed, and the cells were resuspended in 100 μl of PAAG (PAA, 0.2% pig IgG [wt/vol]) containing the amount of directly labeled MAb that would just saturate the surface of the cells. After this incubation, the cells were washed with 1 ml of PAA and spun at 6,000 rpm for 5 min in a microcentrifuge (Hermle). The supernatant was removed, and the cells were resuspended in 1 ml of PBS and transferred to 12- by 75-mm tubes for fluorescence-activated cell sorter analysis. As controls, separate reaction mixtures with the unlabeled MAb were measured with a secondary Alexa Fluor 488-conjugated goat anti-mouse IgG(H+L) (diluted 1:400 in PAAG; Molecular probes) to determine that this MAb was bound to the surface. A positive-control experiment was also performed in which the labeled MAb alone was incubated with the cells. If the unlabeled MAb bound to the same epitope as the labeled MAb, then there would be no or low-level fluorescent reactivity associated with the cells. If the unlabeled MAb bound to a different epitope than the labeled MAb, then the level of reactivity associated with the surface would be equivalent to that of the labeled-MAb-only positive-control cells.
OPK assay.
The OPK assay was described previously (5). OPK activity was defined as a ≥50% reduction of the numbers of CFU in the test MAb reaction mixtures compared to the numbers of CFU in reaction mixtures with an isotype MAb control. An assay control tube which contained effector cells, complement, and bacteria but no antibody demonstrated no killing at 2 h compared to the number of CFU at time zero. This indicated that complement alone was not mediating OPK and that the bacteria were not dying or aggregating from assay conditions alone.
Passive immunization and challenge methods.
All animal work was performed in accordance with the Merck Research Laboratories Institutional Animal Care and Use committee guidelines. The MAbs were evaluated using one of three methods of passive protection. In the first method, bacteria were preopsonized ex vivo with MAb prior to lethal injection via the i.p. route (ex vivo method). In this method, a quantity of bacteria (from RPMI medium culture) sufficient to kill six mice (approximately 1 × 109 to 2 × 109 CFU/mouse) was incubated with 800 μg of IgG at 4°C for 1 h with gentle rocking. Bacteria were then pelleted at 1,800 × g in a tabletop Jouan CR422, and any unbound MAb decanted. Antibody-opsonized bacteria were resuspended, using vortexing and gentle repipetting to achieve apparent homogeneity, in 2.4 ml of PBS. An amount of 0.4 ml (one sixth of the amount of bacteria preopsonized) was injected into each of five BALB/c mice. After challenge, each inoculum was quantitated by plating on TSA to confirm that equivalent numbers of CFU were given to all groups of mice. Survival was monitored for 3 days postchallenge.
The second method was a lethal i.v. challenge method. In this method (lethal challenge method), 300 to 500 μg of antibody per mouse was injected via the intraperitoneal (i.p.) route 18 to 24 h prior to lethal challenge with bacteria (from TSA culture, 5 × 108 to 9 × 108 CFU) via tail vein injection. Each experiment was repeated two to five times with groups of 10 or 20 mice and was monitored for 10 days. BALB/c mice were used in these experiments. Data were pooled for survival analysis, and survival statistics performed for the full 10 days postchallenge.
The last model was an indwelling catheter model. ICR mice had catheters (PE50 silicone rubber) surgically implanted into the jugular vein, held in place with sutures and exiting with a port on the dorsal midline of the mouse. Mice were rested for 9 to 11 days after surgery. At 24 h prior to challenge, mice were passively immunized with a single injection of 600 μg of murine MAb 2H2.BE11 administered i.p. At day 0, mice were challenged with clinical isolate S. aureus MCL8538 administered through the tail vein. The inoculum dose was 2 × 105 to 8 × 105 CFU in a 100-μl volume. At 24 h postchallenge, the catheters were harvested and analyzed for bacterial colonization. The presence of bacteria on the catheters was assessed by culturing the entire catheter on TSA. If any sign of outgrowth was observed on the plate, the catheter was scored as culture positive.
MAb serum persistence.
MAb serum persistence experiments were conducted similarly to the lethal i.v. challenge model. However, persistence cannot be evaluated in the lethal model due to the rapid rate of death. Therefore, mice were injected with a sublethal challenge dose of 10% of the dose used for the lethal model. Groups of 22 mice received 0.5-mg doses of either MAb 13C7.BC1 or isotype control MAb 6G6.A8 20 h prior to i.v. bacterial challenge with 5 × 107 CFU of S. aureus Becker. The bacteria used for challenge were cultured on TSA and thus did not express IsdB at the time of challenge. Two animals from each group were sacrificed just prior to challenge (time zero) to determine the MAb levels in the serum at the time of challenge. At 2, 24, 48, 72, and 96 h postchallenge, four mice from each group were sacrificed and serum MAb levels determined by ELISA. MAb serum concentrations were normalized to that at time zero (100%).
Statistical methods.
For comparisons of survival in the ex vivo lethal challenge experiments and catheter clearance in the indwelling catheter experiments, the results of individual experiments were pooled. The proportion of mice surviving or catheters showing protection from colonization was fitted by using the Generalized Linear Model of the GENMOD procedure in SAS (further details provided in Methods in the supplemental material). The results of individual survival experiments with mice in the lethal i.v. model were pooled, and curves were analyzed by using Prism software, choosing the log rank Mantel Cox test statistical method for testing statistical significance. Curves in the MAb persistence experiment were compared using the Generalized Linear Model procedure in SAS.
RESULTS
MAbs to IsdB.
Two immunization protocols were used to generate high titers of antibodies to IsdB in BALB/c mice for the purpose of hybridoma preparation, as described in Materials and Methods. In the first protocol, IsdB-reactive clones were selected based on binding to recombinant IsdB in an ELISA format. Ultimately, only one of three of these MAbs (2H2.B8) recognized IsdB expressed on the surface of bacteria. Therefore, in the second method, hybridomas were screened by using flow cytometry to select those with binding to native IsdB expressed on bacteria. The two separate immunization/selection protocols resulted in a panel of 12 MAbs (Table 1). From the first protocol, three MAbs were obtained; two were isotype IgG1 and one was isotype IgG2a. The nine MAbs obtained from the second protocol were all isotype IgG1. All of the MAbs contained the κ light chain.
TABLE 1.
Murine MAbs to IsdB obtained after two separate immunization/selection protocols
| MAb IgG1 isotype | Isolation protocola | ELISA resultb | IgG2b isotype MAbc |
|---|---|---|---|
| 2H2.B8 | 1 | ++ | 2H2.BE11 |
| 8H6.E11d | 1 | ++ | |
| 7H2.C11 | 1 | ++ | |
| 2E12.A8 | 2 | ++ | |
| 8A8.B4 | 2 | + | |
| 3G11.D5 | 2 | + | |
| 13G11.C11 | 2 | + | 13G11.BF3 |
| 13C7.D12 | 2 | ++ | 13C7.BC1 |
| 1G3.B3 | 2 | ++ | 1G3.BD4 |
| 9H3.E4 | 2 | ++ | |
| 3B7.G8 | 2 | ++ | |
| 3G12.A4 | 2 | ++ |
MAbs were obtained from mice immunized using one of the two methods described in Materials and Methods.
Binding of MAb to recombinant IsdB in ELISA. ++, higher level of specific binding; +, lower level of specific binding.
MAbs were class switched from IgG1 to IgG2b by using limiting dilution as described in Materials and Methods.
8H6.E11 was the only isotype IgG2a MAb; all other MAbs were of the IgG1 isotype.
MAb binding to IsdB.
Side-by-side comparison of MAb binding to recombinant IsdB indicated that while all 12 of the MAbs were reactive in ELISAs, they fell into two distinct categories. One group of nine MAbs (2H2.B8, 8H6.E11, 7H2.C11, 2E12.A8, 13C7.D12, 1G3.B3, 9H3.E4, 3B7.G8, and 3G12.A4) bound comparably with higher specific activities to IsdB, and the second set of three MAbs (8A8.B4, 3G11.D5, and 13G11.C11) bound comparably with lower specific activities than the first group (Table 1). Representative binding curves are shown in Fig. S3 in the supplemental material. All of the MAbs bound to recombinant IsdB in Western blot analysis (data not shown). Ten of the 12 MAbs bound to surface-expressed, native IsdB on S. aureus, as demonstrated by using flow cytometry (data not shown). Two MAbs (8H6.E11 and 7H2.C11; protocol 1) did not bind to surface-expressed IsdB and were not characterized further. Also, two MAbs with low expression levels (3B7.G8 and 3G12.A4; protocol 2), were only available in small quantities due to the low production of the hybridomas. These two were not characterized further. The eight remaining MAbs were characterized for epitope binding and functional activity. Three methods were employed to determine if the MAbs recognized similar or distinct epitopes.
IsdB mutein binding analysis.
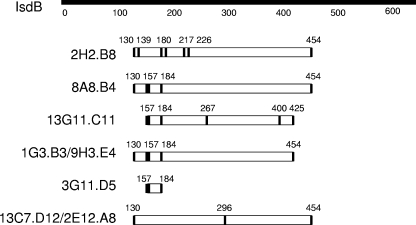
The IsdB-specific MAbs were able to recognize recombinant IsdB in Western blot analysis (data not shown). Therefore, in an attempt to characterize the IsdB binding domains of the MAbs, a series of recombinant mutated IsdB constructs with cumulative mutations were made and were analyzed using Western blots. (See Western binding results and mutein sequences in Tables S2 and S3 in the supplemental material). The muteins were screened with the eight MAbs, and binding with all eight antibodies to multiple muteins was observed in this procedure. As the number of mutations increased, the number of MAbs able to bind decreased until all binding was lost. The eight antibodies were grouped according to their binding profiles (Table 2). Four of the antibodies resolved into two groups (1G3.B3 paired with 9H3.E4, group II, and 2E12.A8 paired with 13C7.D12, group IV) and one MAb had a unique binding profile (3G11.D5, group III), while the three remaining antibodies (2H2.B8, 8A8.B4, and 13G11.C11, group I) had profiles that were similar but not identical to each other.
TABLE 2.
Western blot analysis of MAb binding to IsdB muteins
| MAb | Groupa | Specific amino acid point mutations which cumulatively disrupted binding of MAb to the mutein |
|---|---|---|
| 2H2.B8 | I | 130, 139, 180, 182, 217, 226, 454 |
| 8A8.B4 | I | 130, 157-159, 184, 454 |
| 13G11.C11 | I | 157-159, 184, 267, 400, 425 |
| 1G3.B3 9H3.E4 | II | 130, 157-159, 184, 454 |
| 3G11.D5 | III | 157-159, 184 |
| 13C7.D12 2E12.A8 | IV | 130, 296, 454 |
Grouping for each MAb was determined by Western blot analysis of IsdB mutein binding.
For each antibody, muteins which were recognized in Western analysis were compared to those for which binding was lost. The unique amino acids which contributed to lack of binding (Table 2) were mapped on the full-length protein (also see the supplemental material). These regions potentially contained the MAb binding sites (Fig. 1). An evaluation of binding regions by mutein analysis determined that seven of the eight MAbs required a large, similar portion of the antigen to bind effectively (amino acids [aa] 130 to 454 for 2H2.B8, 8A8.B4, 2E12.A8, 13C7.D12, 1G3.B3, and 9H3.E4 and aa 157 to 425 for 13G11.C11). This implies either that the MAbs bind to discontinuous epitopes that would be dependent upon the antigen conformation or that the mutein analogs have different conformations that shield the respective epitopes. During Western blotting, muteins were presumably denatured; however, it is known that some epitopes are resistant to denaturing during this process and may even renature during electroblotting onto nitrocellulose. Also, antibodies which recognize their epitopes even weakly may bind during Western blotting due to the presence of highly concentrated antigen (2). Therefore, antibodies recognizing linear and/or discontinuous regions of the muteins can potentially recognize and bind to their epitopes in a Western blot format. Compared to that of the other MAbs, the binding of MAb 3G11.B3 was restricted to a smaller portion of the antigen, aa 157 to 184.
FIG. 1.
Potential MAb binding sites on IsdB. IsdB-specific MAbs were reacted with IsdB muteins using Western blotting. MAb binding was disrupted by the accumulation of amino acid mutations in the muteins (Table 2). Amino acids required for binding, as defined by the amino acid mutations which lead to MAb loss of binding, are indicated above the bars. The potential binding site for each MAb is lined up below the corresponding segment of the wild-type IsdB. Vertical lines in the MAb binding site represent amino acid mutations (see Table 2).
IsdB epitope binding analysis by SPR.
Mutein binding analysis could distinguish MAbs with similar binding specificities but did not predict which MAbs might compete for binding to the same IsdB epitope. Therefore, the eight MAbs were compared for competition in binding to recombinant IsdB. Pairwise binding comparisons were conducted using real-time SPR. MAbs with overlapping or similar epitopes were determined, and the eight MAbs could be divided into three groups (Table 3) (further description is provided in Table S1 and Results in the supplemental material).
TABLE 3.
Summary of IsdB-specific MAb grouping, based on evaluation of IsdB binding sites
| Grouping method | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Mutein binding by Western blot | 2H2.B8 | 1G3.B3 | 3G11.D5 | 13C7.D12 |
| 8A8. B4 | 9H3.E4 | 2E12.A8 | ||
| 13G11.C11 | ||||
| SPR analysis (exp. 1) | 2H2.B8 | 1G3.B3 | 13C7.D12 | |
| 8A8.B4 | 9H3.E4 | 2E12.A8 | ||
| 3G11.D5 | ||||
| 13G11.C11 | ||||
| SPR analysis (exp. 2) | 2H2.B8 | 1G3.B3 | 13C7.D12 | |
| 13G11.C11 | ||||
| Native IsdB binding by flow cytometry | 2H2.B8 | 1G3.B3 | 13G11.D5 | 13C7.D12 |
| 8A8.B4 | 9H3.E4 | 2E12.A8 | ||
| 3G11.D5 |
S. aureus binding analysis.
Binding of the eight MAbs to IsdB expressed by S. aureus Becker bacteria was measured by flow cytometry. All 10 MAbs from immunization protocol 2 and MAb 2H2 from protocol 1 did bind to the surface of bacteria cultured in iron-limited medium (RPMI medium) but did not bind to bacteria cultured in iron-rich medium (TSB) (data not shown) (15). Pairwise binding was used to determine the binding of the eight MAbs to similar or distinct IsdB epitopes available on the bacterial surface (see an example of the flow analysis data in Fig. S4 in the supplemental material). Each of the eight antibodies was evaluated using the pairwise binding method. If binding of the labeled MAb was competed by 50% or more by the unlabeled MAb, then those MAbs were considered to be in the same group. Based upon the results of these inhibition studies, the MAbs were divided into four separate groups, which bound to presumably distinct regions of IsdB on the surface of the bacteria (Table 3). This grouping is important to determine which MAbs recognize distinct epitopes of IsdB as it is displayed on the bacteria during host infection.
In comparing all methods used to group the MAbs (Table 3), similar results were observed across the four methods. It was determined that several MAbs recognized similar or overlapping epitopes, and two were not as easily categorized. MAbs 2H2.B8 and 8A8.B4 grouped together in the SPR method (experiment 1) as well as the flow cytometry analysis. Although grouped together in the mutein analysis, the two MAbs had similar rather than identical mutein binding patterns. MAbs 13C7.D12 and 2E12.A8 clearly grouped together in three methods. MAbs 1G3.B3 and 9H3.E4 were also grouped together. The binding of MAbs 13G11.D5 and 3G11.D5 was more difficult to define, and they fell into various groups, depending on the method used for analysis.
For further, functional analyses, MAbs representing four distinct binding sites (group I, 2H2; group II, 1G3; and group IV, 13C7), as well as 13G11.D5 (from group III as defined by the flow cytometry method), were evaluated (Table 4).
TABLE 4.
Summary of functional activities of IsdB-specific MAbs
| Isotype | MAb | Groupa | OPK resultb | MAb-conferred protection in the indicated murine challenge modelc
|
||
|---|---|---|---|---|---|---|
| Ex vivo model | Lethal challenge model | Catheter colonization | ||||
| IgG1 | 2H2.B8 | I | + | + | + | nt |
| IgG2b | 2H2.BE11 | I | + | + | + | + |
| 1G3.BD4 | II | − | − | − | nt | |
| 13G11.BF3 | III | − | − | nt | nt | |
| 13C7.BC1 | IV | − | − | + | nt | |
Grouping based on pairwise flow cytometry analysis; each MAb group represents a nonoverlapping epitope based on this method.
OPK is defined as ≥50% killing activity.
nt, not tested.
Evaluation of in vitro functional activity of MAbs.
Almost all of the murine IsdB-specific MAbs were isotype IgG1. To potentially enhance their functional activity in vivo, four of the MAbs were isotype switched to IgG2b, the isotype of murine IgG which has the most complement-fixing activity (28). This yielded a pair of isotypes for each of the four MAbs (Table 1). Based on binding to IsdB in ELISA and flow cytometry analysis of binding to bacteria, the IgG2b isotype of each MAb had binding properties identical to those of the isotype IgG1 MAb (data not shown).
It is believed that clearance of bacteria in vivo is dependent on functional antibodies which opsonize bacteria, leading to killing by neutrophils. A measure of the functional, or opsonic, activity of an antibody can be determined in vitro using an OPK assay. Antibodies from the four MAb groups determined as described above were evaluated for OPK activity. To determine whether isotype switching improved the functional activity of MAbs, one pair (2H2.B8, IgG1, and 2H2.BE11, IgG2b, from group I) was selected for evaluation in vitro. The two isoforms of MAb 2H2 were compared (Table 5) for OPK activity at 100, 50, and 20 μg/ml. The two isoforms of 2H2 had similar activities, although that of 2H2.B8 was higher. Killing mediated by the MAbs resulted in 41 to 61% bacterial survival at 100 μg/ml and 45 to 53% survival at 50 μg/ml, whereas at 20 μg/ml, the killing activity was lost. Three other MAbs (all of the IgG2b isotype, groups II to IV) were compared to 2H2 (Table 5). These IsdB MAbs had low activities in the OPK assay. For the three concentrations of MAbs evaluated, killing activity did not rise above 50%.
TABLE 5.
OPK activities of IsdB-specific MAbsa
| Irrelevant MAb isotype controlb | MAb | Group | No. of CFU (% survival)c after 2 h of incubation with indicated amount (μg/ml)of MAb
|
|||
|---|---|---|---|---|---|---|
| 100 | 50 | 20 | 0d | |||
| 10B4.H4 (IgG1) | 161, 163 | 110, 161 | 113, 111 | 189, 200 | ||
| 2H2.B8 | I | 74, 60 (41) | 58, 64 (45) | 65, 126 (85) | ||
| 6G6.A8 (IgG2b) | 153, 139 | 145, 170 | 94, 89 | |||
| 2H2.BE11 | I | 107, 70 (61) | 88, 79 (53) | 101, 111 (116) | ||
| 1G3.BD4 | II | 139, 178 (109) | 121, 128 (79) | 98, 107 (112) | ||
| 13G11.BF3 | III | 130, 155 (98) | 114, 125 (76) | 92, 83 (96) | ||
| 13C7.BC1 | IV | 98, 147 (84) | 111, 117 (72) | 68, 90 (86) | ||
The OPK reaction mixture was MAb, complement, HL60 cells, and S. aureus Becker bacteria.
10B4H4 and 6G6.A8 murine MAbs were used as non-S. aureus binding isotype controls.
Data are replicates from two identical reaction mixtures. Percent survival is the mean number of CFU from tubes with test MAb divided by the mean number of CFU from tubes with control MAb, times 100, at 2 h. A percent survival value of >100 indicates bacterial growth during the 2-h assay incubation period. Representative data from one of two experiments with equivalent results are shown.
The numbers of CFU at time zero for the assay control (complement, HL60 cells, S. aureus Becker bacteria, and 0 μg MAb) replicates were 122 and 171.
Opsonization and protection studies using the murine ex vivo lethal challenge model.
To investigate whether IsdB-specific MAbs are opsonic in vivo, passive protection experiments were conducted in which S. aureus was preopsonized with MAb alone (without complement). Bacteria used in this model were cultured in such a way as to ensure IsdB expression, that is, under iron-restricted conditions. MAbs from groups I to IV, including both 2H2 isoforms, were compared for activity in this model. When preopsonized with either 2H2.B8 or 2H2.BE11, but not an isotype-matched control MAb, mice were protected from a lethal challenge dose of staphylococci (Table 6). MAbs 13C7.BC1, 1G3.BD4, and 13G11.BF3 had much less functional activity than 2H2 (Table 6) in this model. In general, the lack of efficacy in this ex vivo opsonization model correlated with the lack of OPK activity in the in vitro assay for these MAbs. Based on the ex vivo experimental results, both isoforms of MAb 2H2 provided protection in vivo, while the other three MAbs tested did not. All of the IsdB-specific MAbs bound to S. aureus RN4220 used in this study, as demonstrated by using flow cytometry (data not shown). Therefore, the differences in protective activity were not due to gross differences in binding to the bacteria.
TABLE 6.
Ex vivo opsonization and protection by IsdB-specific MAbsa
| Irrelevant MAb isotype control or nonprotein control | MAb | Group | No. of tests | Total no. of mice surviving (n = 5/test) | Total % of mice surviving |
|---|---|---|---|---|---|
| 10B4.H4, IgG1 | 8 | 4/40 | 10 | ||
| 2H2.B8 | I | 8 | 39/40 | 98b | |
| 6G6.A8, IgG2b | 5 | 2/25 | 8 | ||
| 2H2.BE11 | I | 3 | 14/15 | 93b | |
| 1G3.BD4 | II | 1 | 1/5 | 20c | |
| 13G11.BF3 | III | 1 | 2/5 | 40c | |
| 13C7.BC1 | IV | 2 | 0/10 | 0c | |
| PBS | 3 | 3/15 | 20 |
The bacterial challenge strain was RN4220. The challenge dose was 2 × 109 to 4 × 109 CFU bacteria.
Comparison of test MAb to control (P < 0.01).
Comparison of test MAb to control (P > 0.09).
Protection studies using the murine i.v. lethal challenge model.
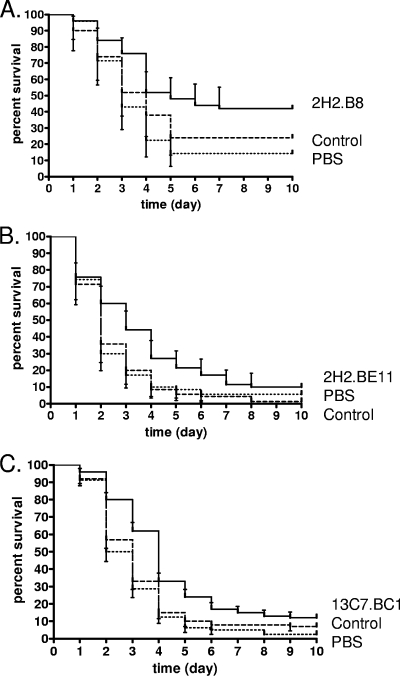
IsdB-specific MAbs from groups I, II, and IV were evaluated for protective efficacy in a lethal i.v. challenge model. Group III MAb 13G11.BF3 appeared to be functionally equivalent to group II and IV MAbs and was not tested further. In pilot studies, MAb doses of from 0.05 to 1.0 mg were tested. A dose of 0.3 to 0.5 mg of MAb was found to confer optimal survival in this model (data not shown). A PBS arm was included to determine if the irrelevant MAb had activity in the model, as injecting protein into BALB/c mice can nonspecifically activate the innate immune system. A small but statistically significant survival enhancement (P < 0.03) was observed when either 2H2.B8 (IgG1) or 2H2.BE11 (IgG2b) was administered to mice prior to i.v. challenge, compared to the rates of survival with the administration of irrelevant isotype control MAbs (Fig. 2A and B, respectively). Passively administered MAb 13C7.BC1 also mediated a small but statistically significant (P < 0.01) survival enhancement in this model, compared to the survival rate with the administration of an irrelevant isotype control MAb (Fig. 2C). This protection was equivalent to the protection afforded by the two 2H2 MAbs. Interestingly, this result was not predicted by data from the ex vivo model or the in vitro OPK activity of 13C7.BC1. MAb 1G3.BD4 (IgG2b) did not enhance survival of mice in this model (P = 0.29; data not shown). This finding corresponds to the MAb's lack of efficacy in the ex vivo model and its lack of significant in vitro OPK activity. In all cases, PBS groups had survival rates equivalent to those of the MAb control groups.
FIG. 2.
Survival of BALB/c mice passively immunized to IsdB and then challenged with S. aureus. Mice were injected i.p. with 0.3 to 0.5 mg of IsdB-specific MAb (solid line), irrelevant isotype control MAb (dashed line), or PBS (dotted line) 20 to 24 h prior to i.v. injection of S. aureus Becker bacteria, as described in Materials and Methods. Data shown are the mean at each time point (error bars show 95% confidence intervals). (A) Mice (five independent experiments, a combined total of 50 per group) were injected i.p. with test MAb 2H2.B8, control MAb, or PBS. In the comparison of test MAb 2H2.B8 and the control MAb, survival was enhanced by the test MAb (P < 0.03; log rank Mantel Cox test). (B) Mice (four independent experiments, a combined total of 40 per group) were injected i.p. with test MAb 2H2.BE11, control MAb, or PBS. In the comparison of test MAb 2H2.BE11 and the control MAb, survival was enhanced by the test MAb (P < 0.01; log rank Mantel Cox test). (C) Mice (four independent experiments, a combined total of 40 per group) were injected i.p. with test MAb 13C7.BC1, control MAb, or PBS. In the comparison of test MAb 13C7.BC1 and the control MAb, survival was enhanced by the test MAb (P < 0.01; log rank Mantel Cox test).
IsdB-specific MAb persistence in serum in the murine i.v. challenge model.
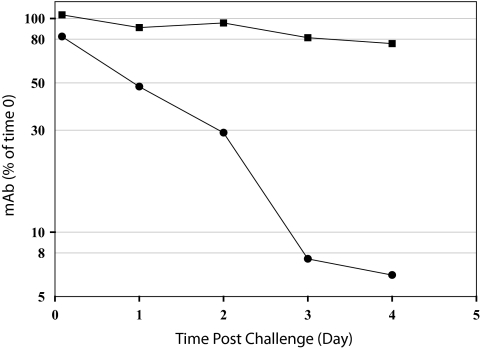
Passive i.p. immunization with IsdB-specific MAbs enhanced survival of mice challenged i.v. with a lethal dose of S. aureus (Fig. 2). After challenge, bacteria express IsdB in the host environment (15). However, it is unclear when the IsdB is available for binding of MAb or how long the MAb persists in the mouse's serum. To determine the length of time IsdB-specific MAb remained in the mouse's blood after challenge, an experiment was performed to measure antibody persistence postchallenge. From this challenge experiment, the half-life of MAb 13C7.BC1 in S. aureus-infected mice was estimated to be approximately 1 day (Fig. 3). In contrast, the half-life of the isotype control MAb was estimated to be greater than 4 days. The half-life for the anti-IsdB MAb in uninfected BALB/c mice was approximately 8 days (data not shown). These data indicated that there was a specific reduction of anti-IsdB MAb in S. aureus-challenged mice. This implies that the IsdB-specific MAb was binding to its target IsdB and being removed from circulation relatively quickly after challenge, presumably as immune complexes.
FIG. 3.
MAb concentrations in blood after S. aureus challenge. Mice (22 per group) were injected i.p. with 0.5 mg of either IsdB-specific MAb 13C7.BC1 (•) or isotype control MAb 6G5.A8 (▪) 20 h prior to an i.v. sublethal challenge with S. aureus Becker. At time zero of the challenge, two mice were sacrificed and blood collected to measure the amount of MAb in the sera at the time of challenge. At each time point indicated, four mice per group were sacrificed and the titer of 13C7.BC1 or 6G6.A8 in sera determined. Titers were calculated based on a standard curve, and concentrations were normalized to those at time zero as described in Materials and Methods. The amount of 13C7.BC1 remaining in the mouse serum after 4 days was statistically less than the amount of control MAb 6G5.A8 remaining in the mouse serum (P < 0.01).
Protection studies with passive immunization in a murine indwelling catheter model.
Although the IsdB-specific MAbs had activity in the lethal models, it was also important to test for activity in a more clinically relevant model. A clinically important source of S. aureus bacteremia is indwelling catheters (21), which can serve as a nidus of infection for bacteria. Therefore, it was of interest to test the IsdB-specific MAbs in a murine indwelling catheter model. Only 2H2 was tested, as it had the best overall activity in the previous models. The aggregate result of four independent experiments indicated that protection of catheters from colonization with MAb 2H2.BE11 was significantly better than with the isotype control MAb 6G6.A8 (Table 7).
TABLE 7.
Murine indwelling catheter model
| Irrelevant MAb isotype control | MAb | Group | No. (%) of culture-negative cathetersa
|
||||
|---|---|---|---|---|---|---|---|
| Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | Totalb | |||
| 6G6.A8, IgG2b | 1/4 (25) | 3/8 (38) | 4/10 (40) | 0/9 (0) | 8/31 (25) | ||
| 2H2.BE11 | I | 3/4 (75) | 6/8 (75) | 4/10 (40) | 4/9 (44) | 17/31 (54) | |
Number of culture-negative catheters obtained in four independent passive transfer experiments in the murine indwelling catheter model.
Comparison of experimental group to control group (P = 0.019).
DISCUSSION
Preclinical and clinical data indicate that immunization with intact whole bacteria induces high immune titers to staphylococcus but does not confer protection from S. aureus disease (10, 17). Immunization with intact bacteria in previous studies may have failed due to preparation of the bacteria in iron-rich media. Staphylococci cultured in this manner may lack important virulence and pathogenic antigens which are upregulated in the low-iron environments of the host. Vaccination with bacteria lacking these virulence factors could lead to an inappropriate or insufficient immune response. Particularly important among the antigens expressed in vivo are the iron-regulated surface determinant proteins, IsdA to IsdH (27, 32). It has recently been demonstrated that immunization with IsdB leads to protection in murine models of S. aureus infection (15, 31). The expression of this antigen occurs under conditions of iron limitation, which is important during growth in vivo. Growth in iron-rich medium, however, does not lead to expression of IsdB. Since little is known about the protective, or opsonic, immune response to IsdB, our study was undertaken to investigate IsdB-specific antibodies which may recognize protective epitopes.
A panel of 12 IsdB-specific MAbs was selected for investigation. All 12 of the MAbs recognized IsdB in Western blot and ELISA analysis. Several methods were utilized to distinguish and group eight of the MAbs. Mutein binding, competition for captured IsdB by SPR analysis, and competition for binding to surface-expressed IsdB by flow cytometry analysis were all utilized in this effort. Each of these methods is informative, but each has its technical drawbacks which could lead to false conclusions if used in isolation. For example, mutein binding analysis may give misleading results due to the potential denaturation of appropriate conformational epitopes by the sodium dodecyl sulfate used in polyacrylamide gel electrophoresis. This could lead to lack of binding due to denaturation of important binding sites. SPR analysis of binding competition for captured IsdB may be misleading due to epitope overlap with the capture antibody, steric hindrance between the antibodies, or large differences between antibody affinities. Additionally, using a MAb to capture IsdB may constrain the antigen to a particular conformation and prevent binding by a second MAb. Pairwise binding to bacteria as evaluated by flow cytometry may result in misleading data for the same reasons described for pairwise binding in SPR. Importantly, the methods utilized yielded, in general, similar information. The combination of the complementary techniques provides higher confidence than a single technique alone. MAbs could be grouped into sets which recognized similar or overlapping epitopes. For example, MAb 2H2.B8 appeared to recognize the same epitope as MAb 8A8.B4. MAb 1G3.B3 appeared to recognize the same epitope as 9H3.E4, and 2E12.A8 and 13C7.D12 bound to similar epitopes. Alternatively, MAbs with distinct epitopes could be recognized by competition for IsdB binding, e.g., MAb 2H2.B8 and 13C7.D12 bound noncompetitively.
The mutein binding analysis indicated that most of the MAbs required a large portion of the IsdB to successfully recognize their binding epitopes (aa 130 to 454 or 156 to 425). This implies that the MAbs recognize discontinuous epitopes or that a large portion of the antigen is necessary to form the correct three-dimensional structure required for binding. Two 125-aa NEAT (“near transporter”) domains are contained within this region of the IsdB protein (9, 25). The NEAT domains mediate hemoglobin binding by IsdB and other members of the Isd family (9, 25). The appropriate conformation of the NEAT domains (aa 140 to 269 and 337 to 462) (25) may be integral for IsdB MAb recognition and binding. Disruption of the biological function of IsdB, which is binding of hemoglobin and import of heme (27), could increase the efficacy of IsdB antibodies, in addition to their opsonizing activity. More work will be necessary to investigate whether the IsdB MAbs can block hemoglobin or heme binding to IsdB.
In terms of potential functional activity, the most relevant method of antibody grouping analysis was perhaps the pairwise binding to IsdB on the surface of bacteria. This method should best reflect which epitopes are exposed on IsdB constrained by expression on the cell surface and sterically hindered by surrounding surface-expressed antigens. IsdB is covalently bound by its C terminus through linkage to the pentaglycine cross bridges of the peptidoglycan in the cell wall (20). Therefore, most accessible on surface-bound IsdB would be its NEAT domain-containing N-terminal portion for binding of MAbs. Based upon flow analysis of pairwise binding, there are four dissimilar epitopes recognized by the eight MAbs evaluated. These epitopes were defined by MAbs 2H2.B8, 1G3.B3, 13G11.D5, and 13C7.D12. Importantly, MAb grouping by this method was similar to the results mapped by mutein binding and SPR binding analysis.
Four MAbs were evaluated for functional activity by in vitro OPK, and MAb 2H2.B8 had the highest activities in this assay, with 59% killing at 100 μg/ml and 55% killing at 50 μg/ml. The isotype-switched 2H2.BE11 had similar activities, with 49% killing at 100 μg/ml and 47% killing at 50 μg/ml. Therefore, when whole serum is used as a source of complement, the isotype of the 2H2 MAb is not critical for OPK activity in this assay. Complement component C3 can be activated by either the classical or the alternative pathways when all of the complement components are available, as in whole serum. C3 is the complement component thought to be most critical for S. aureus opsonization (6). The other MAbs tested had less activity in this assay, and none of them reached 50% killing of bacteria. The level of in vitro OPK activity mediated by IsdB MAbs against S. aureus is much less than that demonstrated for MAbs to S. aureus capsule polysaccharide type 8 (CPs8) (5). Using a similar OPK assay, we demonstrated 70% killing with 100 μg/ml and 60 to 70% killing with 10 and 1 μg/ml of MAb 8E8, a CPs8-specific murine MAb. The robust OPK activity observed with MAb 8E8 in comparison to that for MAb 2H2 may be due to the number of epitopes available for MAb binding to capsule versus to IsdB. A lower number of MAbs binding to the bacterial surface would reduce the opportunity for phagocyte Fc receptor binding and triggering of uptake and killing in this assay. Therefore, the in vitro OPK assay may not be appropriate or sensitive enough to measure the functionality of IsdB-specific MAbs.
Murine challenge models were used to define the protective efficacy of the IsdB-specific MAbs. Similar to results in the in vitro OPK assay, 2H2.BE11 and 2H2.B8 mediated equivalent protective activities in the ex vivo method of challenge (MAb opsonization ex vivo, with lethal challenge i.p.). The other three MAbs tested in the ex vivo model did not mediate the same level of protection as the 2H2 MAb. This corresponds to their poor activities in the in vitro OPK assay. The ex vivo challenge method is basically a means to test for the opsonizing potential of antibodies toward in vitro-cultured S. aureus. Since antibody is in excess during ex vivo opsonization, the affinity of the MAb is not a critical factor. This experiment does not necessarily reflect whether an antibody can bind and opsonize S. aureus when the bacteria has been injected at a site separate from the antibody. This is because the antibody may lack affinity sufficient to opsonize bacteria under in vivo conditions or because the target antigen is not accessible to MAb in vivo.
For a more-stringent test of protective efficacy, the IsdB-specific MAbs were tested in the lethal i.v. challenge model. Three IsdB-specific MAbs were compared for protective activity in this model. A small but statistically significant (P < 0.05) survival enhancement was observed with two isoforms of MAb 2H2 and with 13C7.BC1 but not with MAb 1G3.BD4. Survival enhancement was particularly evident on days 2 to 6. Bacteria from iron-rich medium (e.g., TSA) begin to express IsdB after exposure to low-iron conditions. It is not known how quickly this occurs in vivo, nor when the IsdB is available for MAb binding. However, as demonstrated in the study of persistence in serum, IsdB-specific MAb 13C7.BC1, but not an irrelevant isotype control MAb, was quickly removed from circulation after challenge with S. aureus (half-life of <1 day). The bacteria did not express IsdB at the time of challenge. This implies that the bacteria expressed IsdB quickly after challenge and that MAb successfully bound to IsdB and was removed from circulation. Interestingly, MAb 13C7.BC1 mediated a significant level of protection in the lethal i.v. challenge model, comparable to that of MAb 2H2. This was unexpected in light of the data from both the OPK assay and the ex vivo challenge model, which indicated that 13C7.BC1 did not have functional activity equivalent to that of 2H2. Protection by 13C7.BC1 in the lethal i.v. model could be due to a slightly different expression of IsdB in vivo, allowing better binding of 13C7.BC1 to the bacteria under these conditions, or it could point to a method of protection distinct from classical OPK. Alternatively, it could reflect differences in bacterial clearance through the peritoneum (in the ex vivo model) versus the peripheral circulation (in the i.v. model). The enhancement of mouse survival after passive immunization was not equivalent to the level of survival enhancement observed after active immunization with IsdB (15). Survival after active vaccination with IsdB was perhaps more efficacious due to the polyclonal nature of the anti-IsdB response in the mouse sera and/or the continuing generation of anti-IsdB by B cells during the course of infection (as opposed to a single bolus of MAb in the passive immunization experiments). IsdB-specific MAb had a half-life of only about 1 day in challenged animals. Therefore, at later time points, the MAb concentration could have fallen below the concentration critical for protection.
Finally, MAb 2H2.BE11 was tested in a murine indwelling catheter model. In this model, a catheter was inserted into the jugular vein of the mouse and tied in place with sutures. Bacteria adherent to the catheter begin to form a biofilm by 24 h postchallenge (data not shown). Biofilm formation on indwelling devices is of great clinical concern, since it can serve as a nidus of infection which can lead to seeding of the bloodstream. The aggregate data from four experiments indicated that 2H2.BE11 can protect mice from catheter colonization in this model. This implies that 2H2.BE11 MAb significantly reduced the number of bacteria in the mouse's blood, such that the indwelling catheter was not colonized, and/or that 2H2.BE11 efficiently opsonized bacteria settling on the catheter, such that neutrophils could clear it.
In summary, eight IsdB-specific MAbs were characterized for diversity of IsdB epitope recognition and four for functionality. MAbs could be grouped into three or four groups based on distinct or similar binding sites. MAbs 2H2 and 13C7, defining two noncompeting epitopes, were demonstrated to have in vivo protective efficacy in animal models of infection. The protective efficacy of MAb 2H2.B8 was not enhanced by isotype switching, since the two isotypes performed equivalently in the assays in which they were tested. Two MAbs (1G3.B3 and 13G11.D5) bound both recombinant IsdB and surface-expressed IsdB; however, these MAbs were not efficacious in the murine challenge models. More work is required to further elucidate the nature of IsdB binding by protective versus nonprotective MAbs. Finally, this work supports the finding that IsdB is a potential target for an S. aureus vaccine.
Supplementary Material
Footnotes
Published ahead of print on 24 June 2009.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Arrecubieta, C., I. Matsunaga, T. Asai, Y. Naka, M. C. Deng, and F. D. Lowy. 2008. Vaccination with clumping factor A and fibronectin binding protein A to prevent Staphylococcus aureus infection of an aortic patch in mice. J. Infect. Dis. 198571-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berzofsky, J., and I. Berkower. 1993. Immunogenicity and antigen structure, p. 235-282. In W. E. Paul (ed.), Fundamental immunology. Raven Press, New York, NY.
- 3.Burnie, J. P., R. C. Matthews, T. Carter, E. Beaulieu, M. Donohoe, C. Chapman, P. Williamson, and S. J. Hodgetts. 2000. Identification of an immunodominant ABC transporter in methicillin-resistant Staphylococcus aureus infections. Infect. Immun. 683200-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke, S. R., K. J. Brummell, M. J. Horsburgh, P. W. McDowell, S. A. S. Mohamad, M. R. Stapleton, J. Acevedo, R. C. Read, N. P. J. Day, S. J. Peacock, J. J. Mond, J. F. Kokai-Kun, and S. J. Foster. 2006. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J. Infect. Dis. 1931098-1108. [DOI] [PubMed] [Google Scholar]
- 5.Cook, J., R. Hepler, G. Pancari, N. A. Kuklin, H. X. Fan, X. M. Wang, L. D. Cope, C. Tan, J. G. Joyce, J. Onishi, D. Montgomery, A. S. Anderson, and T. McNeely. 2009. Staphylococcus aureus capsule type 8 antibodies provide inconsistent efficacy in murine models of staphylococcal infection. Hum. Vaccin. 5254-263. [DOI] [PubMed] [Google Scholar]
- 6.Cunnion, K. M., D. K. Benjamin, C. G. Hester, and M. M. Frank. 2004. Role of complement receptors 1 and 2 (CD35 and CD21), C3, C4, and C5 in survival by mice of Staphylococcus aureus bacteremia. J. Lab. Clin. Med. 143358-365. [DOI] [PubMed] [Google Scholar]
- 7.Diekema, D. J., M. A. Pfaller, F. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, M. Beach, and the SENTRY Participants Group. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32S114-S132. [DOI] [PubMed] [Google Scholar]
- 8.Dryla, A., D. Gelbmann, A. von Gabain, and E. Nagy. 2003. Identification of a novel iron regulated staphylococcal surface protein with haptoglobin-haemoglobin binding activity. Mol. Microbiol. 4937-53. [DOI] [PubMed] [Google Scholar]
- 9.Dryla, A., B. Hoffmann, D. Gelbmann, C. Giefing, M. Hanner, A. Meinke, A. S. Anderson, W. Koppensteiner, R. Konrat, A. von Gabain, and E. Nagy. 2007. High-affinity binding of the staphylococcal HarA protein to haptoglobin and hemoglobin involves a domain with an antiparallel eight-stranded beta-barrel fold. J. Bacteriol. 189254-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg, D. P., J. I. Ward, and A. S. Bayer. 1987. Influence of Staphylococcus aureus antibody on experimental endocarditis in rabbits. Infect. Immun. 553030-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregory, S. H., A. J. Sagnimeni, and E. J. Wing. 1996. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J. Immunol. 1572514-2520. [PubMed] [Google Scholar]
- 12.Klein, E., D. L. Smith, and R. Laxminarayan. 2007. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999-2005. Emerg. Infect. Dis. 131840-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klevens, R. M., M. A. Morrison, J. Nadle, S. Petit, K. Gershman, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, E. R. Zell, G. E. Fosheim, L. K. McDougal, R. B. Carey, S. K. Fridkin, and Active Bacterial Core surveillance (ABCs) MRSA Investigators. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2981763-1771. [DOI] [PubMed] [Google Scholar]
- 14.Kohler, G., and C. Milstein. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256495-497. [DOI] [PubMed] [Google Scholar]
- 15.Kuklin, N. A., D. J. Clark, S. Secore, J. Cook, L. D. Cope, T. McNeely, L. Noble, M. J. Brown, J. K. Zorman, X. M. Wang, G. Pancari, H. X. Fan, K. Isett, B. Burgess, J. Bryan, M. Brownlow, H. George, M. Meinz, M. E. Liddell, R. Kelly, L. Schultz, D. Montgomery, J. Onishi, M. Losada, M. Martin, T. Ebert, C. Y. Tan, T. L. Schofield, E. Nagy, A. Meineke, J. G. Joyce, M. B. Kurtz, M. J. Caulfield, K. U. Jansen, W. McClements, and A. S. Anderson. 2006. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect. Immun. 742215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laupland, K. B., D. L. Church, M. Mucenski, L. R. Sutherland, and H. D. Davies. 2003. Population-based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections. J. Infect. Dis. 1871452-1459. [DOI] [PubMed] [Google Scholar]
- 17.Lee, J. C. 1996. The prospects for developing a vaccine against Staphylococcus aureus. Trends Microbiol. 4162-166. [DOI] [PubMed] [Google Scholar]
- 18.Leijh, P. C. J., M. T. Vandenbarselaar, M. R. Daha, and R. Vanfurth. 1981. Participation of immunoglobulins and complement components in the intracellular killing of Staphylococcus aureus and Escherichia coli by human granulocytes. Infect. Immun. 33714-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leijh, P. C. J., M. T. Vandenbarselaar, T. L. Vanzwet, I. Dubbeldemanrempt, and R. Vanfurth. 1979. Kinetics of phagocytosis of Staphylococcus aureus and Escherichia coli by human granulocytes. Immunology 37453-465. [PMC free article] [PubMed] [Google Scholar]
- 20.Mazmanian, S. K., E. P. Skaar, A. H. Gaspar, M. Humayun, P. Gornicki, J. Jelenska, A. Joachmiak, D. M. Missiakas, and O. Schneewind. 2003. Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299906-909. [DOI] [PubMed] [Google Scholar]
- 21.Mermel, L. A., B. M. Farr, R. J. Sherertz, I. I. Raad, N. O'Grady, J. S. Harris, and D. E. Craven. 2001. Guidelines for the management of intravascular catheter-related infections. Infect. Control Hosp. Epidemiol. 22222-242. [DOI] [PubMed] [Google Scholar]
- 22.Middleton, J. R. 2008. Staphylococcus aureus antigens and challenges in vaccine development. Exp. Rev. 7805-815. [DOI] [PubMed] [Google Scholar]
- 23.Otto, M. 2008. Targeted immunotherapy for staphylococcal infections: focus on anti-MSCRAMM antibodies. BioDrugs 2227-36. [DOI] [PubMed] [Google Scholar]
- 24.Peterson, P. K., B. J. Wilkinson, Y. Kim, D. Schmeling, S. D. Douglas, P. G. Quie, and J. Verhoef. 1978. Key role of peptidoglycan in opsonization of Staphylococcus aureus. J. Clin. Investig. 61597-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilpa, R. M., E. A. Fadeev, V. A. Villareal, M. L. Wong, M. Phillips, and R. T. Clubb. 2006. Solution structure of the NEAT (NEAr Transporter) domain from IsdH/HarA: the human hemoglobin receptor in Staphylococcus aureus. J. Mol. Biol. 360435-447. [DOI] [PubMed] [Google Scholar]
- 26.Schaffer, A. C., and J. C. Lee. 2008. Vaccination and passive immunisation against Staphylococcus aureus. Int. J. Antimicrob. Agents 32S71-S78. [DOI] [PubMed] [Google Scholar]
- 27.Skaar, E. P., and O. Schneewind. 2004. Iron-regulated surface determinants (Isd) of Staphylococcus aureus: stealing iron from heme. Microbes Infect. 6390-397. [DOI] [PubMed] [Google Scholar]
- 28.Snapper, C. M., and F. D. Finkelman. 1993. Immunoglobulin class switching, p. 837-863. In W. E. Paul (ed.), Fundamental immunology. Raven Press, New York, NY.
- 29.Spellberg, B., A. S. Ibrahim, M. R. Yeaman, L. Lin, Y. Fu, V. Avanesian, A. S. Bayer, S. G. Filler, P. Lipke, H. Otoo, and J. E. Edwards. 2008. The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus. Infect. Immun. 764574-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spira, G., A. Bargellesi, J. L. Teillaud, and M. D. Scharff. 1984. The identification of monoclonal class switch variants by sib selection and an ELISA assay. J. Immunol. Methods 74307-315. [DOI] [PubMed] [Google Scholar]
- 31.Stranger-Jones, Y. K., T. Bae, and O. Schneewind. 2006. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 10316942-16947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres, V. J., G. Pishchany, M. Humayun, O. Schneewind, and E. P. Skaar. 2006. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J. Bacteriol. 1888421-8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verdrengh, M., and A. Tarkowski. 1997. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect. Immun. 652517-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.