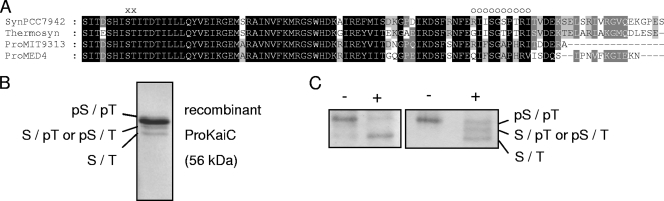
FIG. 1.
(A) Sequence alignment of KaiC C-terminal regions from Prochlorococcus sp. strain MED4 (ProMED4), Prochlorococcus sp. strain MIT9313 (ProMIT9313), Synechococcus elongatus PCC 7942 (SynPCC7942), and “Thermosynechococcus elongatus” BP-1 (Thermosyn), where S431 and T432 (indicated by “x”), the sites of phosphorylation in S. elongatus, are conserved in Prochlorococcus sp. strain MED4 as S427 and T428, respectively. Several residues differ within the A-loop (indicated by “o”) and the C-tail between Prochlorococcus and Synechococcus strains. (B) NanoLC-MS/MS analysis of ProKaiC (purified in the presence of 0.2 mM ATP) revealed the presence of the doubly phosphorylated form pS427/pT428 in the top band and of the singly phosphorylated form in the middle band, respectively. No phosphopeptides could be detected in the faster-migrating band. (C) Dephosphorylation of ProKaiC with λ protein phosphatase (+) of two different preparations (in the presence of 0.2 mM ATP [left] and 0.5 mM ATP [right] during protein purification). After staining, three bands of different mobilities were detected using a high-resolution gel system.