Abstract
Two novel preanalysis sample treatment tools were evaluated in combination with four DNA extraction kits for the selective isolation of bacterial DNA from whole blood. The combination of performing a preanalysis sample treatment and using a larger sample volume increased the detection limit to 50 CFU per ml.
New approaches using molecular technologies are continuously being developed to improve the diagnosis of bloodstream infections. A critical issue in the success of the application of molecular methods is the sample treatment and/or nucleic acid isolation (2). The low concentration of pathogens and the presence of PCR-inhibitory compounds in blood are important challenges that should be dealt with during sample treatment (1, 6, 7, 9, 10). This could be done with so-called preanalysis sample treatment tools, which combine the selective enrichment of bacterial DNA from blood with the integrated, highly efficient removal of PCR inhibitors. The aim of this study was to determine whether the addition of a preanalysis sample treatment to a selective DNA extraction protocol could improve the amplification and detection of bacterial DNA in whole-blood samples.
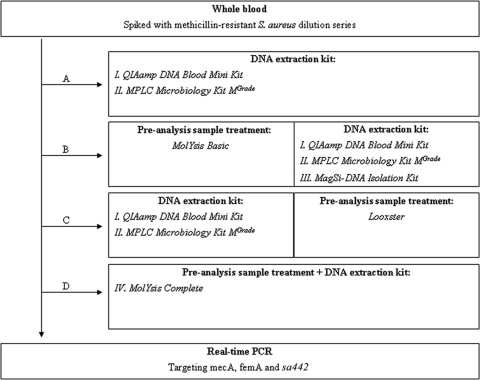
MolYsis Basic (MolZym GmbH & Co. KG, Bremen, Germany) and Looxster (SIRS-lab GmbH, Jena, Germany) were evaluated as novel preanalysis sample treatment tools in combination with the QIAamp DNA blood mini kit (Qiagen GmbH, Hilden, Germany), MagNA Pure LC (MPLC) microbiology kit MGrade (Roche Diagnostics GmbH, Mannheim, Germany), MolYsis Complete kit (MolZym GmbH & Co. KG, Bremen, Germany), and MagSi-DNA isolation kit for blood (MagnaMedics Diagnostics B.V., Maastricht, The Netherlands). A schematic overview of the performed analytical processes for whole blood spiked with methicillin (meticillin)-resistant Staphylococcus aureus is given in Fig. 1. Bacterial DNA extraction was followed by amplification in a multiplex real-time PCR assay targeting three methicillin-resistant Staphylococcus aureus genes (mecA, femA, and sa442) (3). Real-time PCR was set up in a final volume of 50 μl with 2× Absolute QPCR ROX (500 nM) mix (Abgene, Epsom, United Kingdom). Primers and probes were purchased from Sigma-Genosys (Haverhill, United Kingdom) and Applied Biosystems (Nieuwerkerk a/d Ijssel, The Netherlands), respectively. Final reactions contained 0.6 μM of mecA primer and 0.3 μM of femA and sa442 primer; 100 nM, 125 nM, and 150 nM of femA, mecA, and sa442 probe, respectively; and 18.85 μl of template DNA. Optimal thermal-cycling conditions were as follows: initial denaturation at 95°C for 15 min, 42 cycles of denaturation for 15 s at 95°C, and annealing at 60°C for 1 min.
FIG. 1.
Schematic overview of the analytical process for whole-blood samples. (A) DNA extraction with two conventional kits, without preanalysis sample treatment. (B) DNA extraction with two conventional kits and one novel kit, following preanalysis sample treatment with MolYsis Basic. (C) DNA extraction with two conventional kits, followed by a preanalysis sample treatment with Looxster. (D) DNA extraction with a novel kit, which combines all the steps of the preanalysis sample treatment (MolYsis Basic) with a complementary matching DNA extraction protocol.
The detection limit of all DNA extraction procedures was determined and is presented in Table 1. Two conventional DNA extraction kits (QIAamp and MPLC) were tested with and without two different preanalysis sample treatment protocols. Preanalysis sample treatment, with either MolYsis Basic or Looxster, combined with QIAamp extraction, based on a spin-column format, did not result in an increase in detection limit. Both procedures performed equally and were able to detect three target genes at a minimal concentration of 103 CFU per ml, which was analogous to 16 CFU per PCR. Recently, a study by Horz et al. investigated if MolYsis Basic could indeed eliminate human DNA in oral samples to improve the detection of bacterial DNA (4). They found that the use of MolYsis Basic prior to DNA isolation reduced the level of human DNA. However, this effect was accompanied with a partial loss of bacterial DNA. The MPLC kit performed the same as the QIAamp kit, i.e., a detection limit of 103 CFU per ml. The MPLC kit in combination with MolYsis Basic could detect 500 CFU per ml. However, when combined with Looxster, the minimal detectable amount of bacteria was 104 CFU per ml (data not shown). This was most likely due to the incompatibility of the Looxster kit and the MPLC elution buffer, which is essential to the performance of the MPLC kit.
TABLE 1.
Lower detection limits of the methicillin-resistant Staphylococcus aureus real-time PCR assay in relation to preanalysis sample treatment with MolYsis Basic/Looxster and the DNA extraction protocol used
| DNA extraction method | Detection limit (no. of CFU/ml)a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 105 | 104 | 103 | 5 × 102 | 102 | 5 × 101 | 101 | 100 | |
| QIAamp | +++ | +++ | +++ | ++− | −−− | −−− | −−− | −−− |
| QIAamp + MolYsis Basicb | +++ | +++ | +++ | +−− | −−− | −−− | −−− | −−− |
| QIAamp + Looxsterc | +++ | +++ | +++ | ND | −−− | ND | −−− | −−− |
| MPLC | +++ | +++ | +++ | −−− | −−− | −−− | −−− | −−− |
| MPLC + MolYsis Basic | +++ | +++ | +++ | +++ | −−− | −−− | −−− | −−− |
| MagSi-DNA + MolYsis Basic | +++ | +++ | +++ | +−− | −−− | −−− | −−− | −−− |
| MolYsis Complete | +++ | +++ | +++ | +++ | +++ | +++ | ++− | −−− |
Detection represented by a threshold cycle value of <40 is indicated by a +; no detection is indicated by a −. Detection was determined for the three gene targets mecA, femA, and sa442. The data represent results from three independent replicate experiments. ND, not determined.
MolYsis Basic is a preanalysis sample treatment for the removal of human DNA and bacterial enrichment in whole-blood samples.
Looxster is a preanalysis sample treatment for the enrichment of bacterial DNA from total DNA.
The MagSi-DNA kit and the MolYsis Complete kit represented two novel procedures for the targeted isolation of bacterial DNA (Table 1). The MagSi-DNA kit, a novel combination of two sample preparation methods, showed results similar to the results obtained after conventional DNA extraction. The minimal detectable amount of bacteria was 103 CFU per ml. MolYsis Complete was able to achieve bacterial detection at a concentration of 50 CFU per ml and therefore achieved the lowest detection limit compared to those of all the other DNA extraction methods. In this case, the detection of 50 CFU per ml was analogous to 4 CFU per PCR. MolYsis Complete provides a combination of preanalysis sample treatment and targeted DNA extraction containing all the buffers and reagents necessary for human DNA removal, bacteria enrichment, and bacterial DNA extraction. These findings suggest that the combination of two complementary matching sample preparation procedures and the use of a larger volume of blood sample both contributed to a higher level of bacterial detection. Few studies in the past have focused on pathogen detection in whole-blood samples; instead, experiments were performed using bacterial suspensions or clinical sample materials, such as pleural fluid, pus, synovial fluid, and pericardial fluid (5, 8). Zucol et al. evaluated different DNA extraction protocols for whole-blood samples, followed by broad-range, real-time PCR, targeting the 16S rRNA gene in Staphylococcus aureus and Escherichia coli. They achieved the detection of bacterial concentrations of >10 CFU per PCR, which was analogous to 103 CFU per ml (11). Automation, ease of use, duration, and costs of the procedure are each important factors also contributing to the extent of the implementation in diagnostic laboratories. Table 2 shows the detection limit, the duration in time, and the cost per sample obtained for the different DNA isolation protocols. Except for the MPLC kit, which is performed on the automated MagNA Pure instrument, all procedures are performed manually. The extraction methods were all considered easy to perform. The hands-on time for the manual DNA isolation methods combined with sample pretreatment varied between 120 and 240 min.
TABLE 2.
Comparative analysis of the different DNA extraction procedures performed on whole blood spiked with a 10-fold dilution series of methicillin-resistant Staphylococcus aureus
| DNA extraction method | Vba (ml) | Detection limit (CFU/ml) | Costb (€) | Timec (min) |
|---|---|---|---|---|
| QIAamp | 0.2 | 103 | 2.56 | 90 |
| QIAamp + MolYsis Basicd | 0.2 | 103 | 7.06 | 175 |
| QIAamp + Looxstere | 0.2 | 103 | 32.56 | 240 |
| MPLC | 0.1 | 103 | 2.27 | 40 |
| MPLC + MolYsis Basic | 0.2 | 5 × 102 | 6.77 | 125 |
| MagSi-DNA + MolYsis Basic | 0.2 | 103 | n.a. | 130 |
| MolYsis Complete | 1.0 | 5 × 101 | 9.70 | 120 |
Blood volume in ml.
Price per sample for reagents. Not included are plastic wares not provided in the kit. n.a., not available.
Hands-on time for eight samples.
MolYsis Basic is a preanalysis sample treatment for the removal of human DNA and bacterial enrichment in whole-blood samples.
Looxster is a preanalysis sample treatment for the enrichment of bacterial DNA from total DNA.
In conclusion, we investigated whether the addition of a preanalysis sample treatment could improve the efficiency of purifying bacterial DNA from whole-blood samples. The combination of performing a preanalysis sample treatment and using a larger sample volume achieved the detection of only 50 CFU per ml of whole blood (<5 CFU per reaction), emphasizing that the rate of efficiency was attributed to more than one factor. These results confirmed that the efficiency of DNA extraction, especially for clinical samples such as whole blood, is a crucial element in the process of molecular pathogen detection. Ultimately, a combination of optimal sample processing and molecular detection techniques will lead to rapid and accurate pathogen detection for the diagnosis of bloodstream infections.
Footnotes
Published ahead of print on 17 June 2009.
REFERENCES
- 1.Al-Soud, W. A., and P. Radstrom. 2001. Purification and characterization of PCR-inhibitory components in blood cells. J. Clin. Microbiol. 39485-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barken, K. B., J. A. Haagensen, and T. Tolker-Nielsen. 2007. Advances in nucleic acid-based diagnostics of bacterial infections. Clin. Chim. Acta 3841-11. [DOI] [PubMed] [Google Scholar]
- 3.Donker, G. A., R. H. Deurenberg, C. Driessen, S. Sebastian, S. Nys, and E. E. Stobberingh. 2009. The population structure of Staphylococcus aureus among general practice patients from The Netherlands. Clin. Microbiol. Infect. 15137-143. [DOI] [PubMed] [Google Scholar]
- 4.Horz, H. P., S. Scheer, F. Huenger, M. E. Vianna, and G. Conrads. 2008. Selective isolation of bacterial DNA from human clinical specimens. J. Microbiol. Methods 7298-102. [DOI] [PubMed] [Google Scholar]
- 5.Klaschik, S., L. E. Lehmann, A. Raadts, M. Book, J. Gebel, A. Hoeft, and F. Stuber. 2004. Detection and differentiation of in vitro-spiked bacteria by real-time PCR and melting-curve analysis. J. Clin. Microbiol. 42512-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreader, C. A. 1996. Relief of amplification inhibition in PCR with bovine serum albumin or T4 gene 32 protein. Appl. Environ. Microbiol. 621102-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morata, P., M. I. Queipo-Ortuno, and J. de Dios Colmenero. 1998. Strategy for optimizing DNA amplification in a peripheral blood PCR assay used for diagnosis of human brucellosis. J. Clin. Microbiol. 362443-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rantakokko-Jalava, K., and J. Jalava. 2002. Optimal DNA isolation method for detection of bacteria in clinical specimens by broad-range PCR. J. Clin. Microbiol. 404211-4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith, K., M. A. Diggle, and S. C. Clarke. 2003. Comparison of commercial DNA extraction kits for extraction of bacterial genomic DNA from whole-blood samples. J. Clin. Microbiol. 412440-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson, I. G. 1997. Inhibition and facilitation of nucleic acid amplification. Appl. Environ. Microbiol. 633741-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zucol, F., R. A. Ammann, C. Berger, C. Aebi, M. Altwegg, F. K. Niggli, and D. Nadal. 2006. Real-time quantitative broad-range PCR assay for detection of the 16S rRNA gene followed by sequencing for species identification. J. Clin. Microbiol. 442750-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]