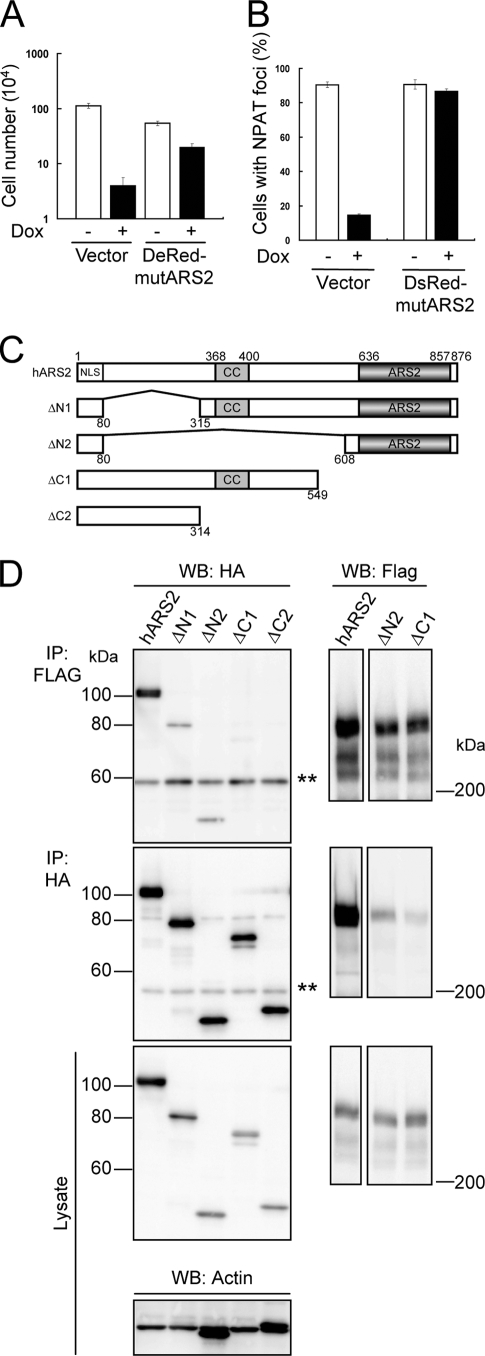
FIG. 5.
ARS2 interacts with FLASH through its ARS2 domain. (A) KB cells with Tet-On shARS2 were infected with an expression vector encoding DsRed-Monomer-mutARS2 (DsRed-mutARS2) or an empty vector (Vector), plated at 1.25 × 103 cells/well in 24-well plates, and cultured with 1 μg/ml Dox for 9 days. Cell numbers were quantified by the trypan blue exclusion method using a hemocytometer. Representative data from three independent experiments, all performed in triplicate, are shown. Error bars denote ± standard errors of the means. (B) KB cells described in panel A were treated with (+) or without (−) 1 μg/ml Dox for 6 days, and NPAT was stained with anti-NPAT Ab. Cells were observed under a confocal fluorescence microscope, and the percentages of cells with NPAT foci (n > 100) were determined. Representative results from three independent experiments are shown. Error bars denote ± standard errors of the means. (C) A graphical overview of full-length hARS2 and various deletion mutants of hARS2. Numbers of amino acid residues in hARS2 are indicated. CC, coiled-coil domain; ARS2, ARS2 domain. (D) 293T cells were transfected with an expression vector encoding HA-hARS2, HA-ΔN1 ARS2, HA-ΔN2 ARS2, HA-ΔC1 ARS2, or HA-ΔC2 ARS2 together with that encoding mFLASH-FLAG3. After 48 h, ARS2 and mFLASH immunoprecipitates (IP) obtained with anti-HA Ab and anti-Flag agarose resin, respectively, were analyzed by Western blotting (WB) with anti-HA and anti-Flag Abs. Actin also was detected as a loading control. Representative results from three independent experiments are shown. Double asterisks indicate the heavy chain of Ab.