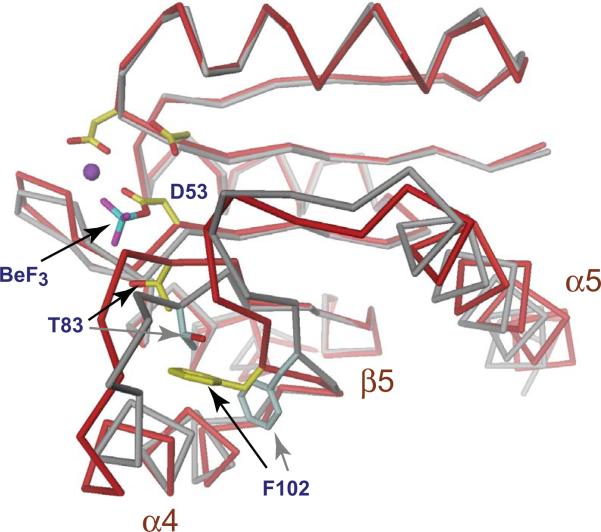
Figure 2. Structural changes upon activation of the response regulator receiver domain.
The structure of the phosphorylated (red) and unphosphorylated (grey) Rec1 domain of PleD is shown to demonstrate the conformational changes imposed upon activation of receiver domain switches [7,66]. The acidic pocket is shown with the phosphoryl-acceptor residue Asp53, a Mg2+ ion (magenta) and a BeF3 moiety. Two additional highly conserved sites, a hydroxyl-containing residue (Thr83) at the C-terminus of β4 and an aromatic residue (Phe102) in β5 are indicated. Upon phosphorylation of the active site aspartate the relative orientations of these residues change from an inactive “outward” conformation (grey) to the active “inward” conformation (yellow). Movement of this molecular lever results in subtle conformational changes that are localized primarily to the α4-β5-α5 face of the receiver domain surface.