Figure 5.
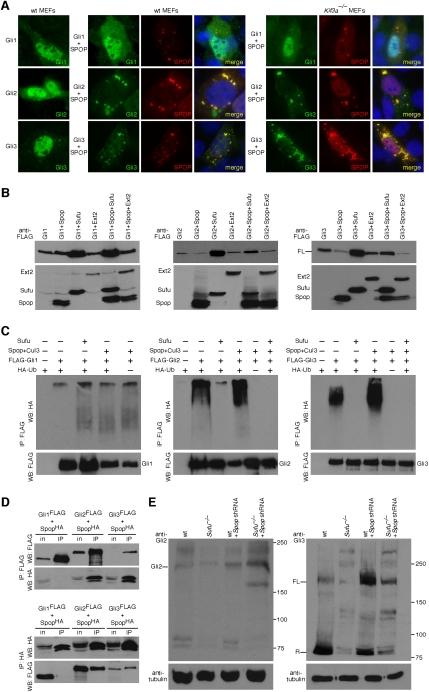
Mouse Spop colocalizes with Gli proteins and antagonizes Sufu in controlling Gli protein levels. (A) Double immunostaining of MEFs transfected singly with Flag-tagged Gli1, Gli2, or Gli3 or cotransfected with Myc-tagged Spop using Flag and Myc antibodies against Flag-tagged Gli1, Gli2, or Gli3 (green) and Myc-tagged Spop (red). Both cytoplasmic and nuclear staining of Gli1, Gli2, and Gli3 was detected. Punctate Spop immunoreactivity in the nucleus and cytoplasm was evident, consistent with previous reports (Hernandez-Munoz et al. 2005). Immunoreactivity of Gli2 and Gli3 (and not Gli1) was reduced when coexpressed with Spop, and Gli2/3 distribution extensively overlaps with Spop, particularly in the cytoplasm. Loss of the primary cilium in Kif3a−/− MEFs has no effect on the subcellular distributions and interactions of Gli1, Gli2, Gli3, and Spop. (B) Western blots of lysates derived from HEK 293T cells expressing Flag-tagged Gli3 singly or in combination with Flag-tagged Spop, Sufu, or Ext2 probed with anti-Flag antibodies. Lack of apparent Gli3 processing in cultured cells has been previously reported (B Wang et al. 2000). Coexpression of Gli2 or Gli3 with Sufu notably enhanced Gli2 and Gli3 protein levels. In contrast, coexpression of Gli2 or Gli3 with Spop (but not the control protein Ext2) significantly reduces Gli2 and Gli3 protein levels, which can then be restored when Sufu is coexpressed. We noticed that reduction in Gli2 protein levels is not as dramatic as Gli3 when Spop is coexpressed. Gli1 or Ext2 protein levels are unaffected when Spop is overexpressed (data not shown). α-Tubulin serves as the loading control (data not shown). (C) Western blot of immunoprecipitated Gli1, Gli2, and Gli3 (epitope-tagged with one copy of Flag) to detect polyubiquitinated Gli proteins. Spop promotes ubiquitination of Gli2 and Gli3 but not Gli1; Gli2 and Gli3 ubiquitination is abolished when Sufu is coexpressed. (WB) Western blot. (D) Western blot of immunoprecipitated Gli1, Gli2, and Gli3 (epitope-tagged with one copy of Flag) to detect physical interaction with Spop (epitope-tagged with one copies of HA) from HEK 293T lysates. Spop physically associates with Gli2 and Gli3 but not Gli1. (in) Input; (IP) immunoprecipitation. (E) Western blots of lysates derived from wild-type and Sufu−/− MEFs and wild-type and Sufu−/− MEFs expressing Spop shRNA probed with anti-Gli2 and anti-Gli3 antibodies. Efficient knockdown of Spop was verified by semiquantitative RT–PCR (data not shown). Gli2 and Gli3 levels are partially restored in Sufu−/− MEFs when Spop is knocked down, consistent with a model in which Sufu and Spop antagonize each other in regulating Gli2 and 3 (but not Gli1) protein levels. Lack of complete rescue of Gli protein levels could be attributed to the presence of additional mammalian Spop homologs (e.g., Spop-like and Tdpoz proteins) (Huang et al. 2004). (FL) Full-length; (R) repressor.