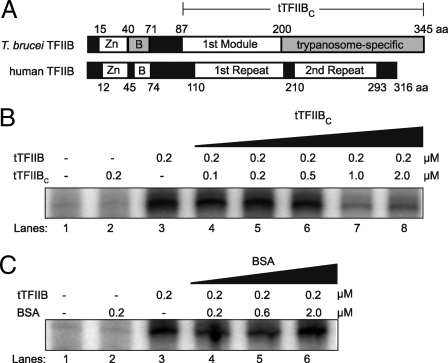
Fig. 1.
Primary structure and function of tTFIIB and tTFIIBC. (A) Schematic of T. brucei and human TFIIB amino acid sequence alignment. The Zn ribbon and the first module/first repeat are well conserved between trypanosomes and humans. The B finger and the trypanosome-specific/second repeat are not well conserved (gray background). tTFIIBC is 259 residues long, comprising the first module and the trypanosome-specific region (bracketed). (B) Activity of tTFIIB and tTFIIBC in in vitro SL RNA transcription assays. Transcription activity in tTFIIB-depleted extracts (lane 1) could not be restored by 0.2 μM tTFIIBC (lane 2), whereas tTFIIB restored maximally at that concentration (lane 3). tTFIIBC maximally inhibited restoration by tTFIIB when added in 5-fold molar excess (lanes 4–8). (C) In control assays, BSA did not restore transcription (lane 2) and did not inhibit restoration by tTFIIB (lanes 4–6).