Abstract
Increases in arousal and activity in anticipation of a meal, termed “food anticipatory activity” (FAA), depend on circadian food-entrainable oscillators (FEOs), whose locations and output signals have long been sought. It is known that ghrelin is secreted in anticipation of a regularly scheduled mealtime. We show here that ghrelin administration increases locomotor activity in nondeprived animals in the absence of food. In mice lacking ghrelin receptors, FAA is significantly reduced. Impressively, the cumulative rise of activity before food presentation closely approximates a Gaussian function (r = 0.99) for both wild-type and ghrelin receptor knockout animals, with the latter having a smaller amplitude. For both groups, once an animal begins its daily anticipatory bout, it keeps running until the usual time of food availability, indicating that ghrelin affects response threshold. Oxyntic cells coexpress ghrelin and the circadian clock proteins PER1 and PER2. The expression of PER1, PER2, and ghrelin is rhythmic in light–dark cycles and in constant darkness with ad libitum food and after 48 h of food deprivation. In behaviorally arrhythmic-clock mutant mice, unlike control animals, there is no evidence of a premeal decrease in oxyntic cell ghrelin. Rhythmic ghrelin and PER expression are synchronized to prior feeding, and not to photic schedules. We conclude that oxyntic gland cells of the stomach contain FEOs, which produce a timed ghrelin output signal that acts widely at both brain and peripheral sites. It is likely that other FEOs also produce humoral signals that modulate FAA.
Keywords: circadian rhythms, oxyntic gland, clock genes, food anticipatory activity, food-entrainable oscillator
Daily fluctuations of CNS arousal and activity require energy output and reflect the need for energy input. Here, we investigate mechanisms that constitute the intersection among circadian time, eating behavior, CNS arousal, and metabolic state. The body uses an endogenous circadian timing system, termed “food-entrainable oscillators” (FEOs), to predict the availability of food. These activate food-seeking behaviors and enable the synthesis and secretion of enzymes necessary for digestion before mealtime. For regularly scheduled daily meals, the behavioral manifestation of this timing mechanism is the expression of food anticipatory activity (FAA), reflected in an increase in activity several hours before the appearance of food. Food anticipatory behavior provides an experimentally tractable window for exploring phenomena associated with anticipatory behavior and the regulation of eating. Many historical lines of evidence converge to indicate that a circadian rather than homeostatic mechanism controls FAA (1). Among these, even when food is withheld for several days and all other environmental conditions are constant, FAA occurs at the time of day that meals had been available previously. FAA survives ablation of the suprachiasmatic nucleus, indicating that this behavior does not require the master clock in the hypothalamus.
Understanding the nature and localization of FEOs has been both controversial and elusive (2–4). In the search for a nervous system site, it remains unresolved whether FEOs lie at one locus or in a network of multiple loci, and whether they are localized to the CNS or also include peripheral nervous system elements. Multiple food-entrainable circadian oscillators have been discovered in the brain and periphery (5), stimulating the search for the localization and identification of FEOs regulating FAA. Many brain areas have been implicated as the neural locus of FEOs, but each of such claims has been challenged (1, 6). Most recently, the dorsomedial hypothalamus (DMH) has been designated a site of FEOs, based on both an unbiased search for brain region(s) that exhibit a rhythmic expression of the Period genes (7) and on site-specific effects of a viral vector containing the Bmal1 gene (8, 9). However, other work challenges such an interpretation because complete DMH ablations do not eliminate FAA (10). Although ablation studies suggest that there is no single neural locus for FEOs, the data indicate several brain sites where food-derived signals influence FAA. Examination of FOS expression and local cerebral glucose utilization points to involvement of a dynamic circuit (11, 12). Also, the earliest sign of behavioral arousal preceding a change in meal time, measured by FOS expression, occurs in the ventromedial hypothalamus (VMH), suggesting that this hypothalamic brain region contributes to the increased activity seen in anticipation of food (13).
Prior searches for the peripheral loci of FEOs involved adrenalectomy (14), subdiaphragmatic vagotomy (15), and capsaicin-induced vagal deafferentation (16), none of which abolish FAA. In fact, the possibility of a peripheral locus was all but dismissed after the demonstration that rhythmic expression of Period1-luciferase in esophagus, stomach, liver, and colon remained nocturnal during total food deprivation, indicating that these oscillators are not self-sustained (17). Although such studies lead to the unsatisfactory conclusion that FEOs and FAA survive ablation of each one of the many brain regions and peripheral organs tested, our experiments take into account the foregoing data and lead to a very different conceptualization: we envision a network of CNS sites at which timed secretion of ghrelin and other signals could modulate FAA. We define FEOs and their output signal by requiring that they meet the following criteria: the signal must (i) antecede mealtime, (ii) stimulate activity in the absence of food deprivation, (iii) promote eating behavior, and (iv) elimination of the FEO output signal or its receptor should eliminate or attenuate FAA. The FEOs that produce this putative signal should be (v) under circadian control, (vi) rhythmic in constant photic and nutrient conditions, and (vii) be entrained to the timing of food presentation. Given the evidence that some mutant mice lacking molecular components of circadian clock exhibit FAA (18), the presence of known clock genes/proteins is not a requirement for FEOs. Nevertheless, circadian rhythmicity of clock genes within FEOs provides evidence of the presence of oscillators.
In the present studies, we explore the possibility that ghrelin-secreting cells of the stomach oxyntic glands are FEOs. Ghrelin, a 28-amino acid endogenous ligand for growth-hormone secretagogue receptor (GHSR) surges before mealtime (19–21). The numerous E-box elements, known targets of circadian clock proteins, present in the promoter region of the ghrelin gene (22) likely play a role in the timing of ghrelin synthesis. Furthermore, plasma levels of ghrelin fluctuate diurnally, with a peak in the day and a trough at night (23), and exogenous ghrelin administration stimulates eating (24).
Results
Peripheral Ghrelin Administration During the Day Increases Anticipatory Activity and Food Intake.
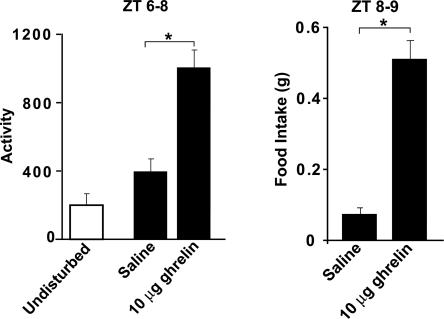
Although ghrelin is known to promote eating, a key question is whether it induces the increased activity that precedes mealtime associated with FAA. To assess the effects of ghrelin on activity in the absence of food deprivation, we injected ad libitum-fed mice with saline or 10 μg of ghrelin i.p. at zeitgeber time 6 (ZT6: lights on at ZT0, off at ZT12) and removed their food from ZT6 to ZT8. General locomotor activity and subsequent food intake were increased after ghrelin treatment. Although it was not quantified, the activity of the animals appeared to be food-oriented, because the animals were seen digging in the bedding on the side of the cage where the food hopper was located. Control undisturbed mice showed little activity at that time of day (Fig. 1). Thus, ghrelin stimulates both activity or arousal and feeding responses.
Fig. 1.
Effect of administration of ghrelin on general activity and food intake. Control animals were not injected or disturbed. Experimental animals were given either saline or ghrelin at ZT6, and food was not available from ZT6 to ZT8. (Left) The amount of activity during the 2-h interval from ZT6 to ZT8. (Right) Food intake in the injected animals from ZT8 to ZT9. *, P < 0.05.
FAA Is Diminished in Ghrelin Receptor Knockout Mice.
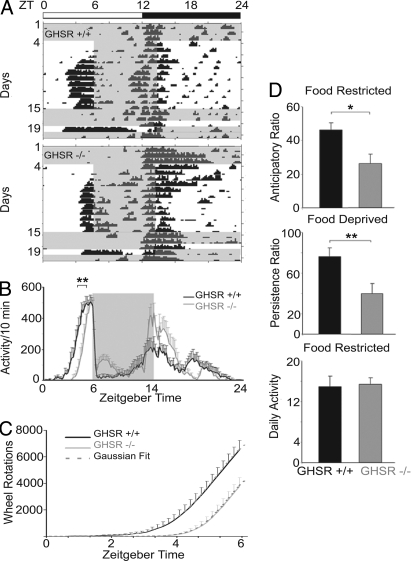
To examine the role of ghrelin in FAA, we tested ghrelin receptor knockout (GHSR−/−) and control (GHSR+/+) mice maintained in a 12:12 h light–dark (12:12 LD) schedule, with food access restricted to 8 h—from ZT6 to ZT14—for 12 days. Next, mice were fed ad libitum for 3 days, then food-deprived for 24 h. We found that GHSR−/− mice had normal overall daily activity but reduced FAA.
Individual animal activity records (Fig. 2A) and group data (Fig. 2B) indicate that the GHSR+/+ mice started their activity bout significantly sooner than did the GHSR−/− mice. There was a remarkable regularity in the cumulative anticipatory activity of the mice as a function of time. Results from both GHSR−/− and GHSR+/+ mice closely fit a Gaussian, with correlations between each of the 2 sets of data and the fitted Gaussian = 0.99, and indistinguishable from the Gaussian by using the sensitive Kolmogorov–Smirnov test. The data from GHSR−/− mice had the same shape of curve as controls, but the knockouts had lower amplitude and started their activity bout later (Fig. 2C). The daily initiation of the anticipatory response had a go, no-go property in that once an animal started its daily anticipatory bout of running (monitored in 10-min time bins), it continued to run until food appeared (Fig. S1). Further analysis shows that the anticipation ratio during food restriction and the persistence ratio during food deprivation were lower in GHSR−/− than in GHSR+/+ mice (Fig. 2D), with no differences between groups in amount of activity during food restriction. There was no significant change in body weight during the food-restriction period [GHSR−/− weight, 29.6 ± 1.1 g during ad libitum and 29.9 ± 0.9 g after food restriction; GHSR+/+ weight, 30.0 ± 2.4 g ad libitum vs. 28.9 ± 2.4 g after food restriction, F(3,30) = 0.08].
Fig. 2.
Running wheel behavior of wild-type and GHSR−/− mice during ad libitum feeding, food restriction, and food deprivation conditions. (A) The bar above the actograms shows the light–dark cycle; time of food availability is shown in gray. Actograms depict activity of representative GHSR+/+ and GHSR−/− mice during ad libitum feeding (days 1–4), food restriction ZT6–ZT14 (days 4–15), ad libitum food availability (days 15–18), and food deprivation (day 19). (B) Group activity profiles show the amount of wheel running during the last 7 days of restricted feeding in GHSR+/+ (black) and GHSR−/− (gray) mice. The data are plotted in10-min bins (mean ± SEM). **, P = 0.002, difference between GHSR+/+ and GHSR−/− in onset time of activity. (C) Line graph of cumulative wheel-running activity (mean ± SEM) from lights on (ZT0) to time of food presentation (ZT6) shows that GHSR−/− mice (solid gray line) ran 42.4% less than GHSR+/+ (solid black line) mice. Superimposed are the curves derived from the Gaussian function f(x) = e−×2/2/√(2π) (dashed lines). (D) The anticipation ratios during 7 days of restricted feeding (Top) and the persistence ratio (Middle) on the day of food deprivation are shown for GHSR−/− (gray bars) and control GHSR+/+ (black bars) mice, with significant differences between groups. (Bottom) Daily activity during the period of food restriction. *, P = 0.02; **, P = 0.01
Rhythms of Ghrelin, PER1, and PER2 in the Stomach.
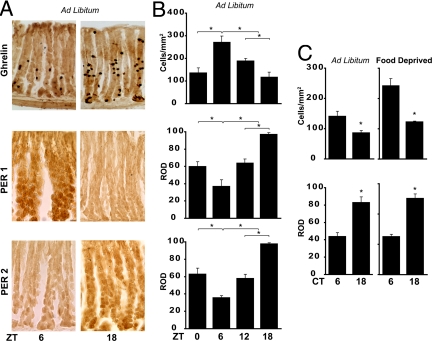
We found that ghrelin-containing oxyntic cells expressed the clock proteins PER1 and PER2 (see Fig. 3 and the high-power view in Fig. S2 A and B), whereas ghrelin adsorption controls and Per1,Per2 mutant mice lacked staining (Fig. S3). We then compared rhythmicity in oxyntic cells in mice housed in a 12:12 LD cycle and fed ad libitum to those in animals kept under constant conditions. Peak and trough expressions of PER1 and PER2 in the oxyntic cells occurred at ZT18 and ZT6, respectively (Fig. 3 A and B). Ghrelin expression is also rhythmic—high during the day and low at night (as in rats; ref. 25)—and is in antiphase to clock protein rhythms. In mice maintained in constant darkness and fed ad libitum or food-deprived for 48 h, ghrelin and PER1 remained rhythmic (Fig. 3C). The results indicate that stomach ghrelin cells contain the circadian molecular machinery. Furthermore, we found that the behaviorally arrhythmic mPer1,mPer2 double-mutant mouse lacked both rhythmic expression of oxyntic cell ghrelin and premeal decrease in stomach ghrelin (Fig. S4).
Fig. 3.
Rhythmicity of ghrelin, PER1, and PER2 in oxyntic cells harvested at various ZTs and CTs in animals fed ad libitum or food-deprived. (A) Photomicrographs of a cross-section through the stomach wall show expression of ghrelin, PER1, and PER2, stained with diaminobenzidine (DAB), at 2 times of day—ZT6 and ZT18—in ad libitum-fed animals. (Scale bar: 20 μm.) (B) Quantification of ghrelin, PER1, and PER2 expression shows daily rhythms in these proteins. Note that peak expression of ghrelin occurs at ZT6, in antiphase with peak expression of PER1 and PER2. (C) Expression of ghrelin and PER1 at CT6 and CT18 in animals housed in DD for 48 h and then either fed ad libitum or food-deprived for 48 h. Note: Control sections are shown in Fig. S3. *, P < 0.05.
Ghrelin, PER1, and PER2 Rhythms Are Controlled by Feeding Time.
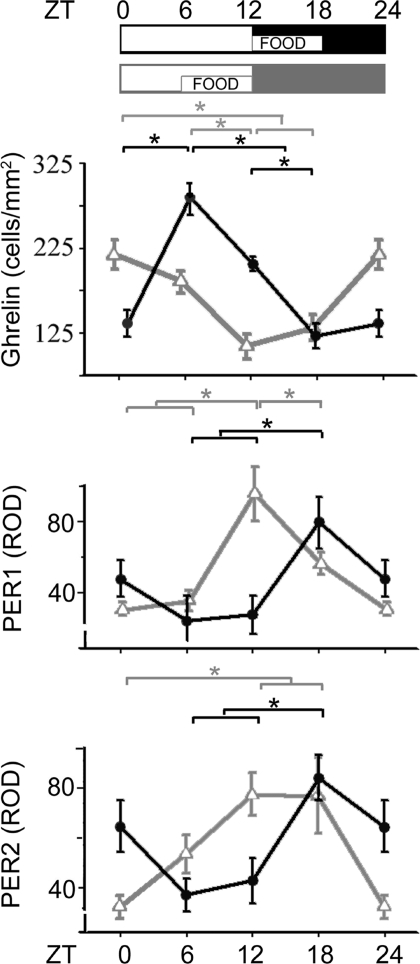
We asked whether ghrelin, PER1, and PER2 rhythms in oxyntic cells are entrained by time of feeding or by the temporal cues of the LD cycle. In mice with food access restricted to the last 6 h of the light period from ZT6 to ZT12 (food-restricted ZT6–ZT12), the phase of the rhythms of all 3 proteins was 6 h earlier than the one of animals fed at ZT12–ZT18 (Fig. 4). The precision of the mechanisms generating the 6-h difference is such that use of 4 time points during 18 h was sufficient to produce a clear result. This indicates that oxyntic cell rhythms are entrained by food-related signals rather than by the LD cycle.
Fig. 4.
The time of food availability determines the phase of ghrelin, PER1, and PER2 rhythms. The schematic shows the 12:12 LD cycle and the time of food availability. The graphs show levels of oxyntic cell expression of ghrelin, PER1, and PER2, respectively, for animals fed at either ZT12–ZT18 (black) or ZT6–ZT12 (gray). *, P < 0.01.
Discussion
Summary of Results.
The results converge to show that stomach oxyntic cells fulfill several essential criteria of an FEO, and the findings provide an avenue for understanding the previous literature on FAA. Ghrelin stimulates both the appetitive (anticipatory locomotor behavior) and the consummatory component (food intake). Administration of ghrelin in the absence of food in a nondeprived animal increases activity/arousal and increases subsequent food intake. In the absence of ghrelin receptors, food anticipatory behavior is diminished. This suggests that ghrelin increases the drive to consume food. Because FAA is not completely abolished, either other ghrelin receptors are activated in this GHSR−/− mouse or, more likely, oxyntic cells are not the only FEOs. Both ghrelin and clock genes are expressed rhythmically within oxyntic cells. The phase of this rhythm is controlled by the time of food availability. Although mice cannot be deprived for many days, in humans, ghrelin release timed to previous mealtimes persists after 1 (26) or 3 (27) days of fasting. In mutant mice lacking a functional circadian clock, ghrelin and clock protein rhythms cease, and the premeal decrease in glandular ghrelin content is abolished. The present series of studies suggest that ghrelin is a signal for FAA and that the stomach oxyntic gland cells are FEOs. Discovery of the brain mechanisms modulating ghrelin effects on activity and eating will further the understanding of this system in the generation of FAA.
Functions and Sites of Ghrelin Action.
As expected for complex physiological systems that coordinate circadian time, arousal, and metabolism, the relevant regulatory endocrine and neuronal functions are distributed. Ghrelin of gastric origin can signal the brain through neural afferents from the periphery or by a direct action in the CNS. Consistent with the former possibility, ghrelin receptors are present in the vagus (28). There are, however, conflicting results on whether or not the vagus plays a necessary role in ghrelin-induced feeding (28, 29). Circulating ghrelin can also reach the brain via circumventricular organs or by crossing the blood–brain barrier (BBB) (30). Neurons of the subfornical organ (SFO), a circumventricular organ lacking a BBB, have ghrelin receptors, and ghrelin affects the electrical activity of SFO neurons (31). The SFO projects to hypothalamic nuclei, such as the arcuate (32), paraventricular, supraoptic nuclei, and lateral hypothalamus (33–35), and these are possible sites for the effects of peripheral ghrelin on the brain.
Ghrelin receptors are found in a number of brain regions and neural circuits implicated in FAA, locomotor activity, and/or feeding. The arcuate nucleus contains ghrelin receptors, and genetic or chemical ablation of agouti-related protein and neuropeptide Y arcuate neurons inhibits ghrelin-induced feeding (36, 37). The nucleus accumbens controls motivated behaviors and reward, including food-associated reward (38). It receives input from the ventral tegmental area (39), which contains ghrelin receptors (40), and has been implicated in ghrelin-induced feeding (41). Lesions of the nucleus accumbens core attenuate FAA (42). The parabrachial–DMH–lateral hypothalamus (PBN-DMH-LH) circuit may serve as another pathway upon which ghrelin acts to regulate FAA. The PBN, DMH, and LH are all critical for the regulation of feeding, body weight, and metabolism. These regions either have ghrelin receptors (40) or express FOS after ghrelin administration (43), and ablation of each of these regions attenuates or abolishes at least some index of FAA (7, 9, 44, 45). The ventromedial nucleus of the hypothalamus is also a good candidate for the effect of ghrelin on FAA. It is the only nucleus (of 16 food- and arousal-related brain sites) to show activation at the start of FAA (13). The VMH contains ghrelin receptors (40, 46–49), and ghrelin application increases the firing rate in a large proportion (≈65%) of VMH neurons in young rats brain slices (Yanagida et al; 50). Moreover, microinjection of ghrelin into the lateral hypothalamus, the medial preoptic area, or the paraventricular nucleus induces wakefulness and eating (51). Finally, ghrelin application to a brain slice phase shifts the suprachiasmatic nucleus (52).
Multiple Potential Signals for FAA.
There are several possible mechanisms that can account for the attenuated FAA in the ghrelin receptor knockout mice, because numerous signals can influence FAA, and many central and peripheral sites may serve as FEOs. Pfluger et al. (53) suggest that novel ghrelin receptors remain to be discovered. Among the hormones that influence food intake, increase before meals, and could signal FAA are apolipoprotein A-IV and corticosterone. Intestinal apolipoprotein A-IV is rhythmically released and is controlled by the timing of meals, with levels increasing before meal onset. Adrenal corticosterone levels are rhythmic and peak in anticipation of feeding (54), but adrenalectomy does not abolish FAA (14, 55). Multiple sites of action for ghrelin compounded by multiple potential signals for FAA argue strongly for the existence of distributed physiological systems—endocrine and neuronal—that regulate the intersection among arousal and activity, metabolic state, and time of day to coordinate them adaptively.
Timing of Anticipatory Activity.
Interestingly, we show that the daily initiation of the anticipatory response has a go, no-go property and that once the mice start their daily anticipatory bout of running, they continue to run until food appears; this finding lends itself to quantitative analysis of its organization and points to possible loci for exploration of its neural basis. The cumulative activity of GHSR−/− and GHSR+/+ mice increases systematically as a function of time since lights on, as well as in advance of the daily appearance of food. This pattern of responses of both GHSR−/− and GHSR+/+ mice closely fits a Gaussian curve (Fig. 2C). The group data indicated that once the animals started their daily running bout, they continued to run until the food appeared. Upon examination of individual animal's data (Fig. S1), the standard error around each data point was revealed to reflect variability in the threshold for arousal and initiation of activity. Because the binomial distribution of yes/no choices of a discrete random variable approximates—with large Ns of choices—the Gaussian, we note that the results are consistent with the speculation that the mechanisms underlying these data include a large number of individual neuronal go, no-go decisions with an increasing proportion of go decisions as feeding time draws near. The excellent fit of the data to a binomial distribution suggests that the decision to activate this appetitive behavior can be understood as a series of repetitive binary choices in which the probability of a positive decision is only about half as large in the ghrelin knockout animal.
Conclusion
The present results integrate information across several levels of analysis to show that stomach gland oxyntic cells are loci of FEOs. These cells contain the machinery that constitutes an FEO: They bear circadian clock genes and ghrelin as their timed output signal. Ghrelin affects both activity (in the absence of food) and eating behavior. In the absence of the circadian clock genes Per1 and Per2, ghrelin is no longer rhythmically expressed, and the premeal glandular decrease is abolished. In the absence of the ghrelin receptor, FAA is diminished but not lost, arguing for the existence of other FEOs and output signals. At the mechanistic level, it remains to be determined how gastric clock genes regulate ghrelin synthesis/secretion and how/where ghrelin acts in the CNS to increase arousal related to food anticipation in the CNS.
With respect to medicine and public health, the results point to a role for the stomach in regulating the timing of meals, in promoting anticipatory arousal, and in inducing eating behavior. Obesity has been associated with a failure in the regulation of the timing of food intake in night eating syndrome (56) and compulsive overeating (57). Ghrelin administration triggers appetite, and ghrelin levels in the blood rise before meals and drop afterward (58). People given ghrelin injections feel voraciously hungry, and they eat more buffet-style meals than otherwise (59). In people who diet and lose weight, ghrelin levels are elevated over their predieting baselines. In contrast, people with morbid obesity treated with bariatric surgery produce less ghrelin and more orexigenic gut peptides; they report feeling less hungry, eat less, and lose weight (60). These studies converge with our results to suggest that ghrelin has a role in the anticipation of eating, including timing of meals, and long-term regulation of body weight.
Materials and Methods
Animals and Housing.
Animals were male C57BL/6 mice (Charles River Laboratories) and ghrelin receptor knockout (GHSR−/−) and control (GHSR+/+) mice (gift from Tamas Horvath, Regeneron Pharmaceuticals, Tarrytown, NY) (Fig. S5). They were housed at 21 ± 1 °C in translucent propylene cages (29 × 19 × 12.5 cm) and adapted to a 12:12 LD (300 lux) schedule for 4 weeks before being used in the experiments. To maintain constant conditions, we used constant darkness (DD) with a dim red light (1 lux) and a white-noise generator (91-dB sound pressure level). All animals were cared for in accordance with the Columbia University Institutional Animal Care and Use Committee and Animal Welfare regulations.
Experimental Design.
Monitoring eating, general activity, and wheel running.
To explore the effect of ghrelin administration on activity and food intake (Fig. 1), male C57BL/6 mice weighing 30–36 g (n = 6) were implanted with a transmitter (MiniMitter), and their general activity was monitored by using Datacol 3 software (MiniMitter) (see SI Text). Two weeks later, they were adapted to the procedure with 0.1 mL of saline i.p. (day 1, ZT4; day 2, ZT11; and day 3, ZT0) and placed in a new cage. During the experiment, the mice received (on different days) i.p. injections of either 0.1 mL of saline vehicle or 10 μg (300 nM) of ghrelin in 0.1 mL saline in random order at ZT6, with each animal receiving each treatment twice. Animals were placed in a new cage (to prevent access to spilled food). The food was replaced and weighed at ZT8, and then weighed again at ZT9.
FAA was assessed by using wheel running in GHSR−/− (n = 8) and control GSHR+/+ (n = 9) mice maintained in a 12:12 LD cycle with food ad libitum and housed in cages equipped with a running wheel. Food was first restricted to ZT6–ZT14 for 12 days, then animals were fed ad libitum for 3 days, and finally were food deprived for 24 h. Wheel-running data were collected by VitalView (MiniMitter) and were quantified by Actiview (MiniMitter) and Microsoft Excel. The “anticipatory ratio” and “persistence ratio” were calculated to quantify the differences in FAA between the mouse strain and its persistence under food deprivation (61). The anticipatory ratio was calculated as the “anticipatory activity” (mean number of wheel revolutions in the continuous bout of activity preceding food presentation at ZT6) divided by the mean number of wheel revolutions per day, averaged over the last 7 days of food restriction. Persistence reflects the occurrence of anticipatory activity in the absence of a feeding stimulus, and it reveals whether or not the rhythm is self-sustaining. For each mouse, the persistence ratio on the day of food deprivation was calculated as the number of wheel revolutions in the single activity bout occurring at the time of prior food presentation, divided by the total daily activity. Activity data were analyzed by ANOVA, followed by Tukey test for pairwise comparisons or Student's t test.
Protein rhythms.
To determine whether ghrelin and PER1 and PER2 proteins oscillate with a daily rhythm in an LD cycle, C57BL/6 mice were killed at 4 time points: ZT 0, 6, 12, and 18 (n = 4 per time point). To establish whether rhythmic expression of these proteins is maintained in constant conditions in ad libitum feeding, mice were housed in LD and then in DD for 42 or 54 h before being killed at circadian time (CT) 6 or 8, respectively (n = 4 per time point projected from the LD cycle). To establish whether rhythmic expression is sustained during food deprivation, C57BL/6 mice were maintained as above but were food-deprived for 48 h before being killed at CT6 or CT18, respectively (n = 4 per time point). To determine whether the LD cycle or timing of food intake entrains ghrelin, PER1, and PER2 expression, C57BL/6 mice maintained in a 12:12 LD cycle received food ad libitum for 2 weeks. Then, half of the animals received food from ZT12 to ZT18 (n = 16), and the other half from ZT6 to ZT12 (n = 16) for 10 days. Animals were killed at ZT 0, 6, 12, and 18 (n = 4 per time point per feeding condition). At ZT6 or ZT12, animals were killed before food availability. Stomach sections were stained for ghrelin, PER1, and PER2, and their expression was quantified as described in SI Text.
Supplementary Material
Acknowledgments.
We thank Dr. Peter Balsam, Dr. Matthew Butler, and Mr. Joe Corey for helpful comments on this manuscript. This work was supported by National Institutes of Health Grants NS37919 (to R.S.) and HD-05751 (to D.W.P.) and National Science Foundation Grant DBI320988 (to Barnard College).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906426106/DCSupplemental.
References
- 1.Mistlberger RE. Circadian food-anticipatory activity: Formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 2.Landry GJ, Mistlberger RE. Food entrainment: Methodological issues. J Biol Rhythms. 2007;22:484–487. doi: 10.1177/0748730407307811. [DOI] [PubMed] [Google Scholar]
- 3.Gooley JJ, Saper CB. Is food-directed behavior an appropriate measure of circadian entrainment to restricted daytime feeding? J Biol Rhythms. 2007;22:479–483. doi: 10.1177/0748730407307810. [DOI] [PubMed] [Google Scholar]
- 4.Mistlberger RE, et al. Standards of evidence in chronobiology: Critical review of a report that restoration of Bmal1 expression in the dorsomedial hypothalamus is sufficient to restore circadian food anticipatory rhythms in Bmal1-/- mice. J Circadian Rhythms. 2009;7:3. doi: 10.1186/1740-3391-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendoza J. Circadian clocks: Setting time by food. J Neuroendocrinol. 2007;19:127–137. doi: 10.1111/j.1365-2826.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- 6.Stephan FK. The “other” circadian system: Food as a Zeitgeber. J Biol Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- 7.Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci USA. 2006;103:12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- 10.Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol. 2006;290:R1527–R1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- 11.Poulin AM, Timofeeva E. The dynamics of neuronal activation during food anticipation and feeding in the brain of food-entrained rats. Brain Res. 2008;1227:128–141. doi: 10.1016/j.brainres.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 12.de Vasconcelos AP, et al. Modifications of local cerebral glucose utilization during circadian food-anticipatory activity. Neuroscience. 2006;139:741–748. doi: 10.1016/j.neuroscience.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro AC, et al. Two forces for arousal: Pitting hunger versus circadian influences and identifying neurons responsible for changes in behavioral arousal. Proc Natl Acad Sci USA. 2007;104:20078–20083. doi: 10.1073/pnas.0710096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulos Z, Rosenwasser AM, Terman M. Feeding schedules and the circadian organization of behavior in the rat. Behav Brain Res. 1980;1:39–65. doi: 10.1016/0166-4328(80)90045-5. [DOI] [PubMed] [Google Scholar]
- 15.Comperatore CA, Stephan FK. Effects of vagotomy on entrainment of activity rhythms to food access. Physiol Behav. 1990;47:671–678. doi: 10.1016/0031-9384(90)90076-g. [DOI] [PubMed] [Google Scholar]
- 16.Davidson AJ, Stephan FK. Circadian food anticipation persists in capsaicin deafferented rats. J Biol Rhythms. 1998;13:422–429. doi: 10.1177/074873049801300507. [DOI] [PubMed] [Google Scholar]
- 17.Davidson AJ, Poole AS, Yamazaki S, Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav. 2003;2:32–39. doi: 10.1034/j.1601-183x.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- 18.Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci USA. 2009;106:6808–6813. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugino T, et al. Effects of ghrelin on food intake and neuroendocrine function in sheep. Anim Reprod Sci. 2004;82–83:183–194. doi: 10.1016/j.anireprosci.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Drazen DL, Vahl TP, D'Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: Evidence for a learned response independent of nutrient status. Endocrinology. 2006;147:23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- 21.Frecka JM, Mattes RD. Possible entrainment of ghrelin to habitual meal patterns in humans. Am J Physiol Gastrointest Liver Physiol. 2008;294:G699–G707. doi: 10.1152/ajpgi.00448.2007. [DOI] [PubMed] [Google Scholar]
- 22.Kanamoto N, et al. Genomic structure and characterization of the 5′-flanking region of the human ghrelin gene. Endocrinology. 2004;145:4144–4153. doi: 10.1210/en.2003-1718. [DOI] [PubMed] [Google Scholar]
- 23.Bodosi B, et al. Rhythms of ghrelin, leptin, and sleep in rats: Effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol. 2004;287:R1071–R1079. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- 24.Ueno H, Yamaguchi H, Kangawa K, Nakazato M. Ghrelin: A gastric peptide that regulates food intake and energy homeostasis. Regul Pept. 2005;126:11–19. doi: 10.1016/j.regpep.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez J, Oliver P, Pico C, Palou A. Diurnal rhythms of leptin and ghrelin in the systemic circulation and in the gastric mucosa are related to food intake in rats. Pflugers Arch. 2004;448:500–506. doi: 10.1007/s00424-004-1283-4. [DOI] [PubMed] [Google Scholar]
- 26.Natalucci G, Riedl S, Gleiss A, Zidek T, Frisch H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: maintenance of a meal-related pattern. Eur J Endocrinol. 2005;152:845–850. doi: 10.1530/eje.1.01919. [DOI] [PubMed] [Google Scholar]
- 27.Avram AM, Jaffe CA, Symons KV, Barkan AL. Endogenous circulating ghrelin does not mediate growth hormone rhythmicity or response to fasting. J Clin Endocrinol Metab. 2005;90:2982–2987. doi: 10.1210/jc.2004-1785. [DOI] [PubMed] [Google Scholar]
- 28.Date Y, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 29.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 31.Pulman KJ, Fry WM, Cottrell GT, Ferguson AV. The subfornical organ: A central target for circulating feeding signals. J Neurosci. 2006;26:2022–2030. doi: 10.1523/JNEUROSCI.3218-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gruber K, McRae-Degueurce A, Wilkin LD, Mitchell LD, Johnson AK. Forebrain and brainstem afferents to the arcuate nucleus in the rat: Potential pathways for the modulation of hypophyseal secretions. Neurosci Lett. 1987;75:1–5. doi: 10.1016/0304-3940(87)90065-6. [DOI] [PubMed] [Google Scholar]
- 33.Lind RW, Van Hoesen GW, Johnson AK. An HRP study of the connections of the subfornical organ of the rat. J Comp Neurol. 1982;210:265–277. doi: 10.1002/cne.902100306. [DOI] [PubMed] [Google Scholar]
- 34.Miselis RR. The efferent projections of the subfornical organ of the rat: A circumventricular organ within a neural network subserving water balance. Brain Res. 1981;230:1–23. doi: 10.1016/0006-8993(81)90388-7. [DOI] [PubMed] [Google Scholar]
- 35.Silverman AJ, Hoffman DL, Zimmerman EA. The descending afferent connections of the paraventricular nucleus of the hypothalamus (PVN) Brain Res Bull. 1981;6:47–61. doi: 10.1016/s0361-9230(81)80068-8. [DOI] [PubMed] [Google Scholar]
- 36.Chen HY, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 37.Luquet S, Phillips CT, Palmiter RD. NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides. 2007;28:214–225. doi: 10.1016/j.peptides.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 38.Szczypka MS, et al. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 39.Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 42.Mendoza J, Angeles-Castellanos M, Escobar C. Differential role of the accumbens Shell and Core subterritories in food-entrained rhythms of rats. Behav Brain Res. 2005;158:133–142. doi: 10.1016/j.bbr.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 43.Olszewski PK, Grace MK, Billington CJ, Levine AS. Hypothalamic paraventricular injections of ghrelin: Effect on feeding and c-Fos immunoreactivity. Peptides. 2003;24:919–923. doi: 10.1016/s0196-9781(03)00159-1. [DOI] [PubMed] [Google Scholar]
- 44.Akiyama M, et al. Reduced food anticipatory activity in genetically orexin (hypocretin) neuron-ablated mice. Eur J Neurosci. 2004;20:3054–3062. doi: 10.1111/j.1460-9568.2004.03749.x. [DOI] [PubMed] [Google Scholar]
- 45.Davidson AJ, Cappendijk SL, Stephan FK. Feeding-entrained circadian rhythms are attenuated by lesions of the parabrachial region in rats. Am J Physiol. 2000;278:R1296–R1304. doi: 10.1152/ajpregu.2000.278.5.R1296. [DOI] [PubMed] [Google Scholar]
- 46.Guan XM, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 47.Howard AD, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell V, et al. Comparative distribution of mRNA encoding the growth hormone secretagogue-receptor (GHS-R) in Microcebus murinus (Primate, lemurian) and rat forebrain and pituitary. J Comp Neurol. 2001;429:469–489. doi: 10.1002/1096-9861(20010115)429:3<469::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 49.Yokote R, et al. Molecular cloning and gene expression of growth hormone-releasing peptide receptor in rat tissues. Peptides. 1998;19:15–20. doi: 10.1016/s0196-9781(97)00263-5. [DOI] [PubMed] [Google Scholar]
- 50.Yanagida H, et al. Effects of ghrelin on neuronal activity in the ventromedial nucleus of the hypothalamus in infantile rats: An in vitro study. Peptides. 2008;29:912–918. doi: 10.1016/j.peptides.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 51.Szentirmai E, Kapas L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am J Physiol. 2007;292:R575–R585. doi: 10.1152/ajpregu.00448.2006. [DOI] [PubMed] [Google Scholar]
- 52.Yannielli PC, Molyneux PC, Harrington ME, Golombek DA. Ghrelin effects on the circadian system of mice. J Neurosci. 2007;27:2890–2895. doi: 10.1523/JNEUROSCI.3913-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfluger PT, et al. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. Am J Physiol Gastrointest Liver Physiol. 2008;294:G610–G618. doi: 10.1152/ajpgi.00321.2007. [DOI] [PubMed] [Google Scholar]
- 54.Shen L, et al. Diurnal changes in intestinal apolipoprotein A-IV and its relation to food intake and corticosterone in rats. Am J Physiol Gastrointest Liver Physiol. 2005;288:G48–G53. doi: 10.1152/ajpgi.00064.2004. [DOI] [PubMed] [Google Scholar]
- 55.Stephan FK, Swann JM, Sisk CL. Anticipation of 24-hr feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav Neural Biol. 1979;25:346–363. doi: 10.1016/s0163-1047(79)90415-1. [DOI] [PubMed] [Google Scholar]
- 56.Fairburn C, Cooper Z. The eating disorder examination. In: Fairburn CG, Wilson GT, editors. Binge Eating: Nature, Assessment, and Treatment. New York: Guilford Press; 1993. pp. 317–360. [Google Scholar]
- 57.O'Reardon JP, et al. Circadian eating and sleeping patterns in the night eating syndrome. Obes Res. 2004;12:1789–1796. doi: 10.1038/oby.2004.222. [DOI] [PubMed] [Google Scholar]
- 58.Cummings DE, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 59.Wren AM, et al. Ghrelin enhances appetite and increases food intake in humans. Journal Clinical Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 60.Doucet E. Gastrointestinal peptides after bariatric surgery and appetite control: Are they in tuning? Curr Opin Clin Nutr Metab Care. 2008;11:645–650. doi: 10.1097/MCO.0b013e32830ab9c9. [DOI] [PubMed] [Google Scholar]
- 61.Mistlberger RE, Marchant EG. Enhanced food-anticipatory circadian rhythms in the genetically obese Zucker rat. Physiol Behav. 1999;66:329–335. doi: 10.1016/s0031-9384(98)00311-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.