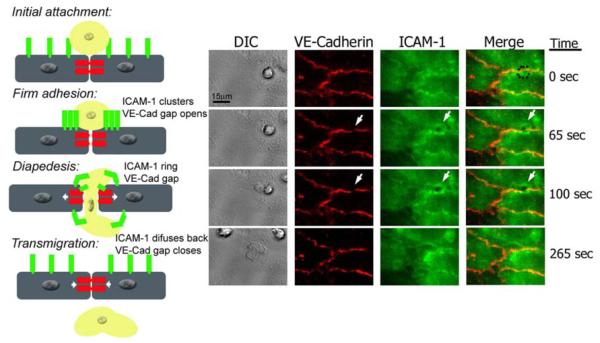
Figure legend 2. Live imaging of endothelial cell VE-Cadherin gaps and ICAM-1 rings dynamics during transmigration.
HUVECs were activated with TNF-α for 4 h and stained with nonblocking mAbs to VE-Cadherin and ICAM-1 directly conjugated in red (Alexa 568) and green (Alexa 488), respectively. Freshly isolated neutrophils were drawn through a parallel plate flow chamber apparatus at 1 dyne/cm2 of shear stress. Three-channel, live-cell digital microscopy of neutrophils and endothelium during the process of transmigration was carried out as described previously (69). At time= 0, the neutrophil approaches the brightly stained cell junction during the initial attachment to the 4-hr TNF-α activated HUVECs. The dynamic behavior of VE-Cadherin and ICAM-1 has not started. At time=65 sec, the neutrophil firmly adheres to the endothelial cells at the junctions, and as VE-Cadherin starts forming a de novo gap, ICAM-1 clusters at the cell-cell junction where the neutrophil is firmly adhered. At t= 100 sec, the neutrophil is undergoing diapedesis, and part of it is still on top of the monolayer, while part of it is underneath. At this point the VE-Cadherin gap reaches its maximum aperture, and ICAM-1 forms a ring-like structure surrounding the transmigrating neutrophil. Finally, at t= 265 sec, the neutrophil has completely transmigrated, and the endothelial cell junction returns to normal: the VE-Cadherin gap is closed, and ICAM-1 is again uniformly distributed on the apical surface of the endothelial cell.