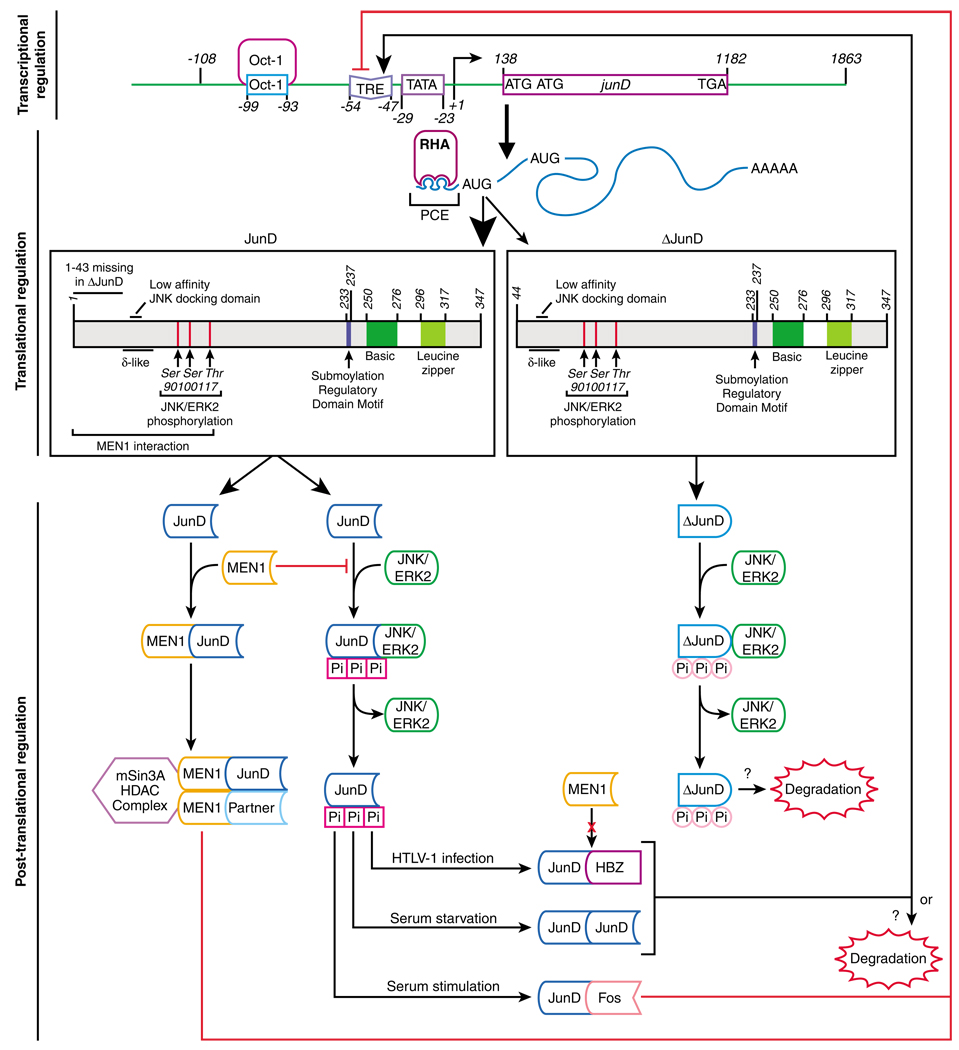
Figure 1.
The regulation of JunD activity is operated by interrelated layers of transcriptional, post-transcriptional and post-translational control. The top panel summarizes general features of the human junD gene structure and its transcriptional regulation. Positions within the promoter region of the octamer-binding transcription factor 1, TPA response element (TRE) and TATA-binding sites are indicated with sequence numbering relative to the transcription start site (+1). The transcription of junD is constitutive and subject to an autoregulatory loop. The middle panel summarizes the structure of the primary transcription product and two translation products. JunD is more abundant than the N-terminal truncated ΔJunD, as indicated by the thicker arrow. The mRNA contains one exon and no introns. Two alternative translation initiation codons are utilized and these mRNA templates contain either a 138-nt (JunD) or a 681-nt (ΔJunD) 5′ UTR. The 5′ UTR forms stem loop structures that that act as a 5′ terminal PCE. PCE interaction with RHA derepresses mRNA translation and is necessary for junD translation. Domains of the JunD protein are indicated: basic and leucine zipper domains, MEN1 interaction region, the δ-like domain, JNK phosphorylation sites and the sumoylation regulatory domain motif. The N-terminus of JunD is truncated in ΔJunD, which profoundly alters the profile of protein–protein interactions. The lower panel summarizes protein–protein interactions that modulate the versatile functional activity of JunD. The N-terminal low-affinity docking site is important for efficient JunD phosphorylation by JNK, as indicated with red squares labeled Pi. ΔJunD, which lacks this domain is not efficiently phosphorylated by JNK, as indicated by the pink circles labeled Pi. Also, ΔJunD does not interact with MEN1. Interaction of JunD with MEN1 inhibits transcription of JunD target genes. MEN1 interaction with JunD has been proposed to be inhibited for JunD-HBZ heterodimers. MEN1 recruits an HDAC complex that renders the JunD dimer a repressor. Phosphorylation by JNK/ERK2 also is inhibited by MEN1. The identity of the dimerization partner of JunD is determined by the cell type and growth conditions. Little is known about the mechanism of JunD protein degradation. ERK, extracellular signal-regulated kinase; HDAC, histone deacetylase; JNK, Jun N-terminal kinase; MEN1, multiple endocrine neoplasia type-1; PCE, post-transcriptional control element; RHA, RNA helicase A; TPA, 12-O-tetradecanoyl-phorbol-13-acetate; TRE, TPA-response element.