Abstract
(−)-Rhazinilam was spontaneously generated from a natural product during isolation. In cultured cells, it causes microtubule bundle formation, like those caused by paclitaxel. With tubulin, (−)-rhazinilam causes formation of an aberrant spiral polymer. Using glutamate and GTP, we developed an assay for spiral formation and applied it to 17 new (±)-rhazinilam analogs with either a modified side-chain or a different size D ring. There was reasonable correlation between spiral formation and inhibition of human MCF-7 breast carcinoma cell growth. Only one side chain analog was as active as (±)-rhazinilam. During these studies, we observed that omitting GTP from the reaction mixture caused a major change in the morphology of the (−)-rhazinilam-induced polymer, with half the observed polymer being microtubule-like and half being spirals. This mixed polymer slowly disassembled at 0 °C, but there was no apparent difference in the lability of the microtubules versus the spirals.
Keywords: tubulin, (−)-rhazinilam, rhazinilam analogs, microtubule morphology, GTP, sedimentation assay, MCF-7 human breast carcinoma cells, spiral morphology of tubulin polymer, vinblastine, glutamate
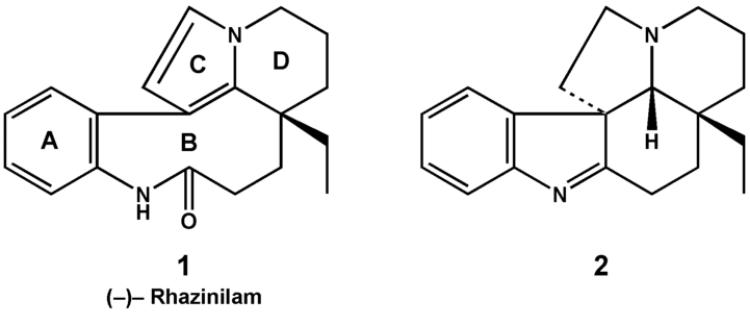
Of all the antimitotic compounds that interact with tubulin, one of the most unusual is (−)-rhazinilam (1). Obtained from a number of plant species of the Apocynaceae family, (−)-rhazinilam was found not to be a true natural product. Instead, the actual natural product (2) rapidly degrades to the biologically active (−)-rhazinilam [1, 2]. Structures of 1 and 2 are shown in Fig. 1.
Fig. 1.
Structures of (−)-rhazinilam (1) and its presumptive precursor 2.
The biological activity of (−)-rhazinilam is as unusual as its origin. Although relatively weak as a cytotoxin for an antitubulin agent, since typically it arrests cell growth with IC50's in the low μM range, (−)-rhazinilam has effects on cells most similar to those of paclitaxel. It causes mitotic arrest and formation of extensively bundled, short microtubules in interphase cells [3]. With microtubule protein1 or purified tubulin, however, (−)-rhazinilam acts more like the vinca alkaloids [4] or the colchicine site drug curacin A [5]. In some studies (−)-rhazinilam inhibits tubulin polymerization [2], while at higher concentrations it causes formation of a morphologically aberrant spiral polymer (sometimes called an “aggregation” reaction) [3]. Paclitaxel, in contrast, causes the hyperassembly of tubulin and microtubule protein into polymers of reasonably normal morphology, as well as tubulin hyperassembly in cells with formation of bundled microtubules [6, 7].
Our studies with rhazinilam began with chemical synthetic work to gain greater SAR understanding of the compound, with the goal of designing a more active analog. There have been a number of previous synthetic efforts aimed at producing more active analogs of rhazinilam, but none has succeeded thus far in producing a compound significantly more cytotoxic than the parent compound [8]. We directed our attention to two features of the rhazinilam molecule, ring D and the side chain attached to the junction of rings B and D (ring nomenclature as in David et al. [9]).
Quantitation of inhibitory compound effects on tubulin assembly is straightforward, and usually turbidimetric analysis of assembly data is used [10]. We initially attempted to apply our standard method to evaluate the inhibitory phase of (−)-rhazinilam's effects on tubulin assembly, but we found that the inhibitory window was too narrow, particularly with many of our newly synthesized analogs. We therefore decided to explore development of a centrifugal method to quantitate (−)-rhazinilam and analog effects on formation of the spiral polymer. We found that spiral formation was so extensive that we could readily determine compound concentrations that caused 50% of the tubulin to form these aberrant polymers.
In evaluating a number of possible reaction conditions, we also examined polymer morphology in the electron microscope. With the reaction condition chosen for quantitative evaluation of analog effects, the polymer induced by (−)-rhazinilam consisted entirely of tubulin spirals. However, in the course of these studies we found that if no GTP were added to the reaction mixture, a mixture of spirals and microtubule-like polymers was formed. As far as we are aware, this represents the first observation of polymer of “normal” morphology induced by (−)-rhazinilam in a biochemical assay with pure tubulin.
Materials and methods
Materials
Synthetic (−)-rhazinilam was a generous gift of Dr. F. Guéritte, Centre National de la Recherche Scientifique, Gif-sur-Yvette, France. The synthesis of the rhazinilam analogs ((±)-rhazinilam and diastereoisomeric compounds 3-19) will be described elsewhere (manuscript in preparation). Purified bovine brain tubulin was prepared as described previously [11]. GTP, from Sigma, was repurified by triethylammonium bicarbonate anion exchange chromatography and was over 99.5% pure at the time of isolation (periodic reevaluation has shown no significant deterioration).
Tubulin assembly assay
Reaction mixtures contained 10 μM (1.0 mg mL−1) tubulin, 0.75 or 0.80 M monosodium glutamate (pH of 2.0 M stock solution adjusted to pH 6.6 with HCl), 4% (v/v) dimethyl sulfoxide as compound solvent, and concentrations of GTP and individual compounds as indicated.
In the centrifugal assay, sample incubation was for 20 min at 30 °, followed by centrifugation for 15 min at 14,000 rpm at room temperature (about 20 °C) in an Eppendorf benchtop centrifuge. Aliquots of the supernatants were removed and their protein content determined by the Lowry method. Aliquots of uncentrifuged reaction mixtures were also evaluated for total protein content of reaction mixtures. The EC50 was defined as the concentration of agent required to remove 50% of the tubulin from the supernatant versus reaction mixtures without compound.
Reactions were also followed turbidimetrically in Beckman DU7400/7500 spectrophotometers, as described in detail elsewhere [10].
Electron microscopy
Aliquots of reaction mixtures were placed on 200-mesh carbon-coated, Formvar-treated copper grids and immediately stained with 5-10 successively applied drops of 1% (w/v) uranyl acetate. Excess stain was wicked from the grids with torn filter paper. The grids were examined in a Zeiss model 10CA electron microscope.
Growth of human breast carcinoma MCF-7 cells
Cells, obtained from the National Cancer Institute drug screening group, were grown in RPMI-1600 supplemented with 10% heat-treated fetal bovine serum. Cell growth was measured by quantitating cellular protein with sulforhodamine B [12]. About 5,000 cells per well were seeded onto a 96-well microtitre plate and allowed to adhere overnight. Drug effects on growth were determined following an additional 48 h incubation at 37 °C in a humidified atmosphere containing 5% CO2. The final dimethyl sulfoxide concentration was 0.5 % (v/v).
Results
Initial evaluation of effects of (−)-rhazinilam on tubulin assembly in 0.8 M glutamate
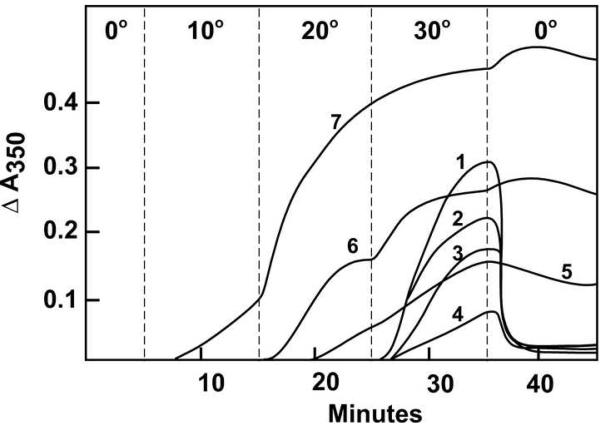
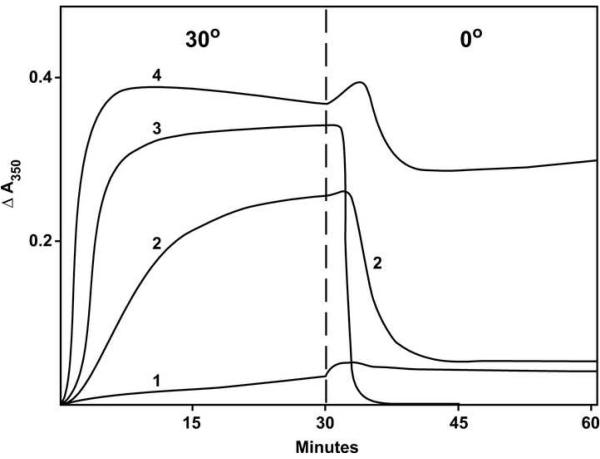
We have used glutamate for quantitative evaluation of inhibitors of tubulin assembly [10], including drugs such as vinblastine, dolastatin 10, and curacin A that cause various aberrant assembly reactions [4, 5, 13]. An initial study (Fig. 2) suggested that this method would be effective with (−)-rhazinilam as well, but the narrow window between the end of the inhibitory effect (3 μM in Fig. 2, curve 4) and the onset of spiral induction (4 μM in Fig. 2, curve 5) proved too narrow to obtain consistent results, particularly with many of the analogs we examined.
Fig. 2.
Effects of varying concentrations of (−)-rhazinilam on glutamate-induced assembly with 0.4 mM GTP. Each reaction mixture contained 10 μM (1.0 mg mL−1) tubulin, 0.8 M monosodium glutamate, 0.4 mM GTP, 4% dimethyl sulfoxide, and concentrations of (−)-rhazinilam as follows: curve 1, none; curve 2, 1.0 μM; curve 3, 2.0 μM; curve 4, 3.0 μM; curve 5, 4.0 μM; curve 6, 5.0 μM; curve 7, 10 μM. The temperature controller was set to the indicated temperatures at the times indicated by the vertical dashed lines to the left of each temperature. In this experiment there was a tubulin-drug preincubation without GTP for 15 min at 30 °C [10].
Development of a centrifugal assay for structure-activity comparisons
We next explored spiral induction as the parameter for comparisons between compounds. The reaction condition we settled upon for drug comparisons was 0.75 M glutamate, 10 μM tubulin, and 10 μM GTP, with 4% dimethyl sulfoxide as the compound solvent and 30 °C as the incubation temperature. Fig. 3 shows a turbidimetric study with increasing concentrations of (−)-rhazinilam. The reaction without drug was minimal (curve 1), and a similar negligible reaction was observed without drug when no GTP was added to the reaction mixture (see below). When we used centrifugation of reaction mixtures to determine the amount of aberrant polymer that was formed, we obtained an EC50 value of 6.6 ± 2 (SD) μM for (−)-rhazinilam (Table 1).
Fig. 3.
Effects of varying concentrations of (−)-rhazinilam on assembly in glutamate with 10 μM GTP. Each reaction mixture contained 10 μM tubulin, 0.75 M monosodium glutamate, 10 μM GTP, 4% dimethyl sulfoxide, and concentrations of (−)-rhazinilam as follows: curve 1, none; curve 2, 2 μM; curve 3, 5 μM; curve 4, 10 μM; curve 5, 20 μM. Incubation was at 30 °C.
Table 1.
Activities of (−)-rhazinilam and analogs as inhibitors of MCF-7 cell growth and inducers of formation of tubulin spirals
| Compound | Spiral formationa | Inhibition of cell growthb |
|---|---|---|
| EC50 (μM) ± S. D. | IC50 (μM) ± S. D. | |
| (−)-rhazinilam | 6.6 ± 2 | 0.97 ± 0.3 |
| (±)-rhazinilam | 17 ± 1 | 1.8 ± 0.7 |
| 3 | 68 ± 4 | 8.6 ± 1 |
| 4 | 56 ± 2 | 9.2 ± 1 |
| 5 | 27 ± 3 | 3.8 ± 1 |
| 6 | 28 ± 2 | 3.2 ± 0.1 |
| 7 | 25 ± 1 | 3.5 ± 1 |
| 8 | 20 ± 1 | 3.7 ± 0.8 |
| 9 | > 100 | > 10 |
| 10 | > 100 | > 10 |
| 11 | 28 ± 4 | 8.1 ± 2 |
| 12 | > 100 | > 10 |
| 13 | > 100 | > 10 |
| 14 | 21 ± 0.8 | 4.2 ± 2 |
| 15 | > 100 | > 10 |
| 16 | 11 ± 2 | 2.2 ± 1 |
| 17 | 47 ± 5 | 4.4 ± 2 |
| 18 | > 100 | > 10 |
| 19 | > 100 | > 10 |
In the spiral formation assay, 0.1 mL reaction mixtures contained varying compound concentrations, 10 μM tubulin, 10 μM GTP, 0.75 M monosodium glutamate, and 4% (v/v) dimethyl sulfoxide. The amount of protein removed by centrifugation without compound was 8 ± 2 %. See text for further details.
In the cell growth assay, the effect of varying compound concentrations on the proliferation of MCF-7 human breast carcinoma cells was determined. See text for further details.
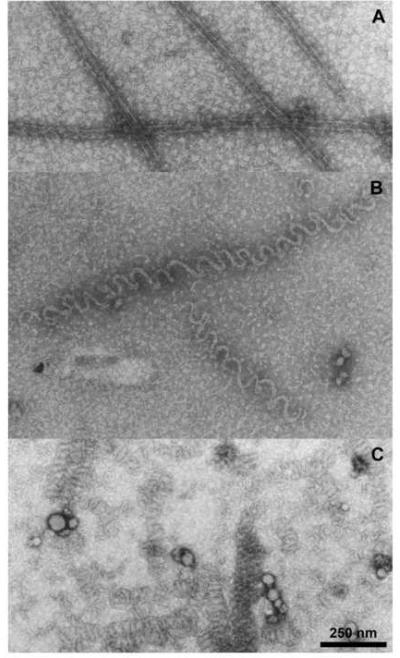
We verified by electron microscopy that the morphology of the (−)-rhazinilam-induced polymer formed in glutamate with purified tubulin was similar to that described previously [3] with microtubule protein. We compared polymers formed without drug (Fig. 4A) with those formed with (−)-rhazinilam (Fig. 4B) or with vinblastine (Fig. 4C). Although not abundant (consistent with the minimal turbidity development shown in Fig. 3, curve 1), scattered microtubules were present in the control reaction mixture (a rare cluster is shown in Fig. 4A). With (−)-rhazinilam, abundant, relatively loose spiral polymers were formed, whereas with vinblastine the typical tight spirals, similar to those reported by many workers [4], were observed.
Fig. 4.
Electron microscopic evaluation of morphology of polymer formed in glutamate with 10 μM GTP. Reaction mixtures contained 10 μM tubulin, 0.75 M monosodium glutamate, 10 μM GTP, 4% dimethyl sulfoxide and either no further addition (A), 10 μM (−)-rhazinilam (B), or 10 μM vinblastine (C). Grids for electron microscopy were prepared after 30 min at 30 °C.
Elimination of GTP from the reaction mixture caused a major change in polymer morphology
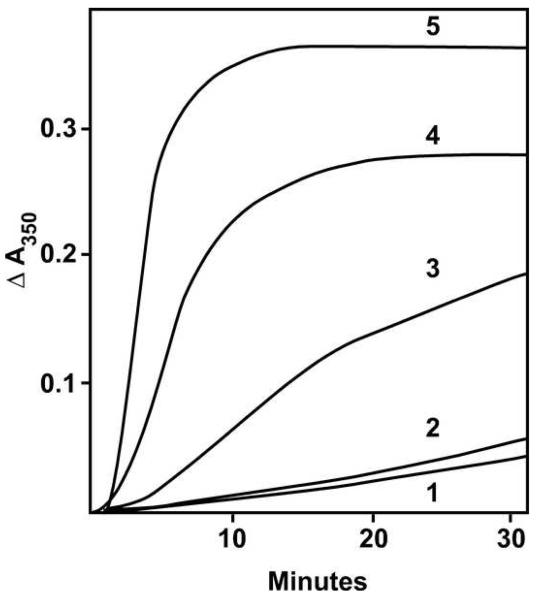
Among the reaction conditions we examined for possible use for determination of EC50 values was 0.75 M glutamate without GTP. While it had an advantage over the 10 μM GTP system described above in that there was no polymer at all formed without drug, as determined by electron microscopy, with (−)-rhazinilam turbidity development was slower than with even a small amount of GTP (Fig. 5, curve 2, 20 μM (−)-rhazinilam, no GTP; cf. Fig. 4, curve 5, 20 μM (−)-rhazinilam + 10 μM GTP). In Fig. 5 we also show typical reactions with 0.4 mM GTP in the absence (curve 3) and presence (curve 4) of 20 μM (−)-rhazinilam. The reaction mixtures represented in Fig. 5 were also subjected to a 0 °C incubation following the 30 °C incubation. The polymer formed with only 0.4 mM GTP (Fig. 5, curve 3) rapidly disassembled in the cold, as described previously [14], while with (−)-rhazinilam + 0.4 mM GTP there was little disassembly (Fig. 5, curve 4). The polymer formed with (−)-rhazinilam in the absence of GTP disassembled slowly in the cold, and the disassembly was not complete, as judged by residual turbidity (Fig. 5, curve 2)..
Fig. 5.
Effect of 20 μM (−)-rhazinilam on assembly in glutamate without GTP and with 0.4 mM GTP. Each reaction mixture contained 10 μM tubulin, 0.75 M monosodium glutamate, 4% dimethyl sulfoxide, and concentrations of (−)-rhazinilam and GTP as follows: curve 1, no GTP and no (−)-rhazinilam; curve 2, no GTP and 20 μM (−)-rhazinilam; curve 3, 0.4 mM GTP and no (−)-rhazinilam; curve 4, 0.4 mM GTP and 20 μM (−)-rhazinilam. At zero time the temperature controller was set at 30 °C. At the time indicated by the vertical dashed line, the temperature controller was set at 0 °C.
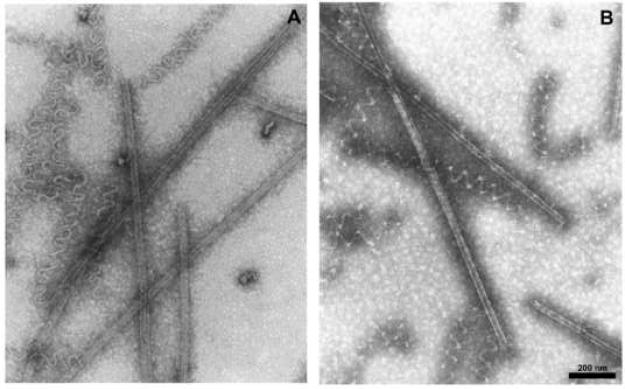
We present these data because of the unexpected electron micrographs we repeatedly obtained with glutamate and without GTP. As shown in Fig. 6A, an electron micrograph prepared after a 30 min incubation at 30 °C, the polymer was composed of a mixture in roughly equal amounts of short open spirals and microtubule-like structures with parallel protofilaments. A similar mixture of structures was observed after only a 5 min incubation, indicating no significant difference in the rate of formation of the two structures. We also examined the remaining polymer after a 30 min incubation at 30 °C followed by 30 min at 0 °C, to determined whether there was differential cold stability of the two morphologically distinct polymers. This did not appear to be the case, as shown in Fig. 6B. We observed no instances in which there were attachments between the two types of polymer, but we cannot exclude fragmentation during grid preparation.
Fig. 6.
Electron microscopic evaluation of morphology of polymer formed in glutamate without GTP. The reaction mixture contained 10 μM tubulin, 0.75 M monosodium glutamate, 4% dimethyl sulfoxide, and 20 μM (−)-rhazinilam. Grids for electron microscopy were prepared after 30 min at 30 °C (A) and after 30 min at 30 °C followed by 30 min at 0 °C.
We have tried many different reaction conditions to shift the balance further or entirely towards microtubules, but we have thus far not succeeded. Among the reaction components we examined were Mg2+ and GDP, microtubule-associated proteins, and other anions that induce tubulin assembly, such as glutarate, aspartate, and succinate. With microtubule-associated proteins and no GTP with (−)-rhazinilam, we observed only spirals. With other assembly inducing anions and no GTP with (−)-rhazinilam, we saw mixtures of microtubules and spirals similar to what was observed with glutamate. The tubulin used in these studies contains a small amount of residual unbound GDP. We observed no significant change in polymer morphology either when additional GDP was added to the reaction mixture or when tubulin freed of unbound nucleotide by gel filtration chromatography [15] was used.
Comparison of the effects of (−)-rhazinilam and the newly synthesized analogs on spiral formation with purified tubulin and on the growth of MCF-7 cells
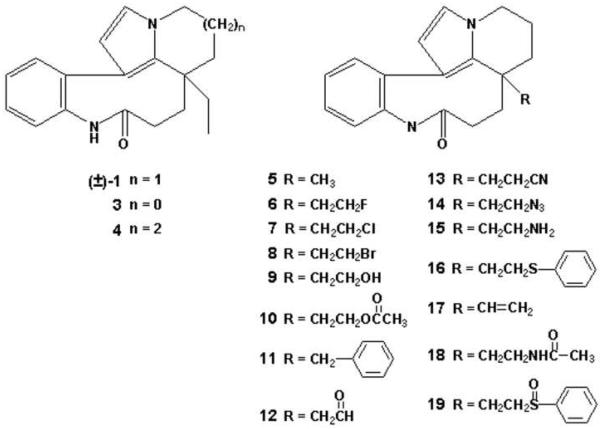
In Table 1 our SAR results are summarized, and analog structures are shown in Figure 7. Our synthetic methods produced racemic mixtures of all compounds, so we also prepared (±)-rhazinilam for comparative purposes. In both biological assays, the latter compound was approximately half as active as (−)-rhazinilam itself, in agreement with the finding of Thoison et al. [2] that (+)-rhazinilam is inactive. We therefore conclude that most likely only one enantiomer is active in each analog preparation and that this active isomer would be twice as active as the racemic mixture. We were successful in preparing one analog, compound 16, that had activity comparable to that of (±)-rhazinilam with both tubulin and the MCF-7 cells. A range of activities was observed in both the cell- and tubulin-based activities with the other analogs. Compounds with less activity than (±)-rhazinilam but activity in both assays above the thresholds selected (IC50's < 10 μM for the MCF-7 cells and EC50's < 100 μM in the spiralization assay) were compounds 3-8, 11, 13, 14, and 17.. The remaining analogs had little activity in either the cell-based or tubulin-based assay (compounds 9, 10, 12, 13, 15, 18, and 19).
Fig. 7.
Structures of racemic rhazinilam analogs (compounds 3-19). Note that (±)-1 is (±)-rhazinilam.
Compounds 3 and 4 were designed to address the questions as to whether the D ring could be contracted from 6 to 5 members (3) or expanded to 7 members (4). Both changes were deleterious to both cytotoxicity, with about a 4-fold increase in the IC50, and to induction of tubulin spirals, with about a 4-5-fold increase in the EC50. Combining either of these changes with either a methyl or benzyl group as the side chain yielded inactive compounds (data not presented). We thus conclude that the size of the D ring affects the affinity of rhazinilam analogs for the tubulin binding site.
Compounds 5-19 were designed to examine the effect of replacing the ethyl side chain with a wide variety of alternate substituents. A significant number of analogs with alternate side chain substitutions had little or no activity (compounds 9, 10, 12, 13, 15, 18, and 19). As noted above, however, compound 16, with a bulky thiophenylethyl group, was as active as (±)-1. The remainder of the active analogs, with the exception of 11 and 17, were essentially identical in their activities with both tubulin and MCF-7 cells. Compounds 5-8 and 14 had IC50's against the cell line of 3.2-4.2 μM, 1.5-2-fold that of (±)-rhazinilam. In the spiralization assay, the values obtained with these compounds (20-28 μM) averaged about 1.4-fold the value obtained with (±)-rhazinilam. Thus, replacing the ethyl side chain with either a methyl, an ethyl halide (F, Cl, or Br) or an ethylazido side chain had relatively small effects on analog activity. Compound 11 was similar to the other compounds in its effects on spiral formation (EC50, 28 μM), but it was half as active with the MCF-7 cells. Its IC50 of 8.1 μM was similar to that of 3 and 4. Compound 17 had activity intermediate between the more active cohort of analogues and the less active 3 and 4.
At this point, no definitive SAR conclusion can be reached regarding the composition of the side chain. Assuming that (±)-rhazinilam represents “full activity,” only an analog with a bulky substituent (16) was fully active with both tubulin and as an antiproliferative agent, while others with bulky side chains were either partially active (11, 14) or inactive (19). Reducing the size of the substituent resulted in partial (5) or total (12) loss of activity. Reducing the ethyl group to an ethylene group (17) led to partial loss of activity. Electronegativity in a substituent had unpredictable results. The ethyl halides (6-8) all had comparable small reductions in activity, but all compounds with oxygen atoms in the side chain (9, 10, 12, 18, 19) were inactive. Similarly, electropositive substituents in the form of compounds containing nitrogen atoms resulted in partial (14) or total (13, 15, 18) loss of activity. The most reasonable interpretation of these results is that specific amino side chains at the binding site on tubulin, unknown at the present time, influence the affinity of the side chain substituent for the protein through a combination of steric factors and hydrogen bonds.
Discussion
Up to now the most puzzling property of (−)-rhazinilam was the contradiction between its effects on cellular microtubules (taxoid-like, induction of assembly) and its biochemical effects on tubulin (vinca-like, inhibition of assembly at lower concentrations and induction of aberrant spiral polymers at higher concentrations). There are numerous possible explanations for this apparent discrepancy, but our results suggest that the primary reason is that the biochemical assays thus far used to study this compound do not represent a good model for intracellular conditions. Other possibilities that now seem less likely are tubulin isotype/post-translational modification differences between brain tubulin and tubulin in cultured cells or intracellular metabolism of (−)-rhazinilam to a different and more active chemical entity. Since synthetic (−)-rhazinilam mimics the cellular and biochemical effects of the natural product-derived (−)-rhazinilam, we can exclude contamination of the latter by a more active compound as the explanation.2
An additional question with (−)-rhazinilam is its binding site on tubulin, which the results of David et al. [3] suggest may be the vinca site. These workers found that both vinblastine and maytansine, a competitive inhibitor of vinca alkaloid binding to tubulin [16-18], inhibited (−)-rhazinilam-induced spiral formation. Higher concentrations of maytansine, which does not appear to induce aberrant tubulin assembly [19], and of vinblastine, with vinblastine spiral formation, prevented binding of (−)-[3H]rhazinilam to tubulin. However, the radiolabeled (−)-rhazinilam was found to have a high affinity only for (−)-rhazinilam-induced spirals and did not bind efficiently to unassembled αβ-heterodimer, to paclitaxel-stabilized microtubules, to residual microtubules remaining following (−)-rhazinilam addition (addition of (−)-rhazinilam to microtubules results in spiral formation at microtubule ends, with only limited microtubule disassembly), or to microtubule ribbons formed in the presence of dimethyl sulfoxide [3]. Finally, David et al. [3] also reported that (−)-rhazinilam inhibited the binding of [3H]vinblastine to tubulin, but we have been unable to reproduce this result.
It is thus difficult to interpret the effects of maytansine and vinblastine unambiguously in terms of binding sites. If (−)-[3H]rhazinilam cannot bind to the αβ-heterodimer, the inhibitory effect of maytansine, an especially potent antitubulin drug, may simply result from failure to form spirals, while the mutually inhibitory effects of vinblastine and (−)-rhazinilam might indicate masking of their respective sites in the morphologically different spiral polymers they induce.
David et al. [3] also reported that the tubulin-colchicine complex formed spirals in the presence of (−)-rhazinilam. Since colchicine binds very tightly to tubulin, this implies that (−)-rhazinilam does not bind in the colchicine site. Consistent with this result, we found no inhibitory effect on the binding of radiolabeled colchicine to tubulin by (−)-rhazinilam.
Nor does it seem likely that (−)-rhazinilam binds in the taxoid site. This analysis is complicated by the much greater affinity of taxoids for microtubules, including preformed microtubules, than for unassembled tubulin [20-24], combined with the fact that inhibitors of microtubule assembly also inhibit tubulin polymerization induced by taxoid site drugs [25, 26]. Thus, high concentrations of paclitaxel can appear to inhibit colchicine or vinblastine binding and vice versa, although the three drugs are generally thought to bind at distinct sites on tubulin (supported by crystallographic evidence [27-29]). However, Takoudju et al. [24] have shown that a radiolabeled taxoid binds to (−)-rhazinilam-induced spirals, with the spirals persisting following addition of the taxoid. Consistent with this finding, we have observed no inhibitory effect of (−)-rhazinilam on the binding of radiolabeled paclitaxel to preformed microtubules (unpublished data).
We raise the question of the (−)-rhazinilam binding site specifically because of our finding that omitting GTP from reaction mixtures can lead to a major shift in polymer morphology. This suggested to us that (−)-rhazinilam might bind in the exchangeable nucleotide site on β-tubulin. We therefore performed experiments with tubulin bearing [8-14C]GDP, instead of nonradiolabeled GDP, in the exchangeable site and measured the radiolabeled nucleotide to tubulin content in polymer pellets induced by adding (−)-rhazinilam, taxol, or UTP + nucleoside diphosphate kinase to the reaction mixture. In all three cases, a similar result was obtained. This would seem to exclude (−)-rhazinilam's effects on tubulin assembly being mediated directly by binding in the exchangeable site.
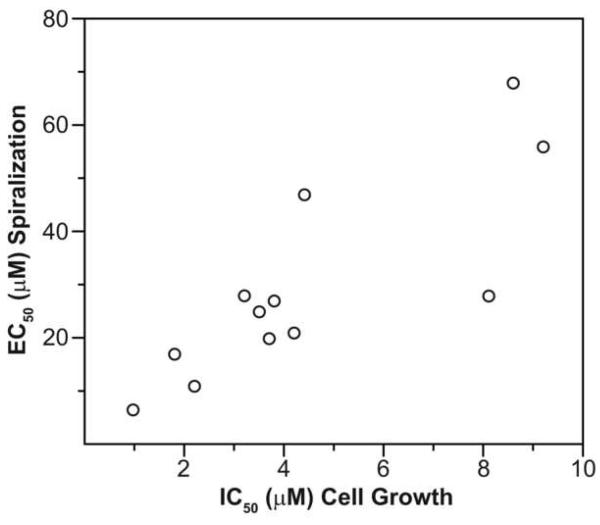
As described here, we designed a quantitative assay for (−)-rhazinilam-induced spiral formation, and we used this assay in conjunction with an antiproliferative assay with MCF-7 human breast carcinoma cells. We found, with two series of analogs that we synthesized to determine effect of the size of ring D and of the side chain substituent, that there was good correlation between the two assays (Fig. 8). With the analogs we prepared, we found that the 6 member ring D of rhazinilam is optimal for activity, and no side chain replacement yielded superior activity to (±)-rhazinilam. Side chain substituents had surprisingly unpredictable effects on activity, and one of the bulkiest replacements for the ethyl side chain, a thiophenylethyl group, yielded an analog (compound 16) with activity equivalent to that of (−)-rhazinilam.
Fig. 8.
Correlation of EC50 data obtained for induction of spiral formation with IC50 data for proliferation of MCF-7 cells. The average values from Table 1 are plotted in the Figure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations used: IC50, the concentration of a compound that causes 50% inhibition of cell growth or of tubulin assembly; EC50, the concentration of a compound that causes removal of 50% of the tubulin in the reaction mixture by centrifugation.
Microtubule protein is the term commonly used to indicate a tubulin preparation prepared from a tissue extract, generally brain tissue, by cycles of assembly and disassembly. Such preparations generally contain 70-80% tubulin plus a variety of additional proteins referred to as microtubule-associated proteins. In this paper we will use the terms “tubulin” and “purified tubulin” interchangeably, referring to a protein preparation from which the microtubule-associated proteins have been removed.
We should note that we have confirmed with synthetic (−)-rhazinilam the observation that the compound causes formation of extensive bundled microtubules in cultured cells.
References
- 1.De Silva KT, Ratcliffe AH, Smith GF, Smith GN. Tetrahedron Lett. 1972;10:913–916. [Google Scholar]
- 2.Thoison O, Guénard D, Sévenet T, Kan-Fan C, Quirion J-C, Husson H-P, Deverre J-R, Chan K-C, Potier P. C. R. Acad. Sc. Paris Série II. 1987;304:157–160. [Google Scholar]
- 3.David B, Sévenet T, Morgat M, Guénard D, Moisand A, Tollon Y, Thoison O, Wright M. Cell Motil. Cytoskel. 1994;28:317–326. doi: 10.1002/cm.970280405. [DOI] [PubMed] [Google Scholar]
- 4.Himes RH. Pharmacol. Ther. 1991;51:257–267. doi: 10.1016/0163-7258(91)90081-v. [DOI] [PubMed] [Google Scholar]
- 5.Hamel E, Blokhin AV, Nagle DG, Yoo H-D, Gerwick WH. Drug Dev. Res. 1995;34:110–120. [Google Scholar]
- 6.Schiff PB, Fant J, Horwitz SB. Nature (London) 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 7.Schiff PB, Horwitz SB. Proc. Natl. Acad. Sci. U.S.A. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baudoin O, Guénard D, Guéritte F. Mini-rev. Org. Chem. 2004;1:333–341. [Google Scholar]
- 9.David B, Sévenet T, Thoison O, Awang K, Païs M, Wright M, Guénard D. Bioorg. Med. Chem. Lett. 1997;7:2155–2158. [Google Scholar]
- 10.Hamel E. Cell Biochem. Biophys. 2003;38:1–21. doi: 10.1385/CBB:38:1:1. [DOI] [PubMed] [Google Scholar]
- 11.Hamel E, Lin CM. Biochemistry. 1984;23:4173–4184. doi: 10.1021/bi00313a026. [DOI] [PubMed] [Google Scholar]
- 12.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, Gray-Goodrich M, Campbell H, Mayo J, Boyd M. J. Natl. Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 13.Bai R, Taylor GF, Schmidt JM, Williams MD, Kepler JA, Pettit GR, Hamel E. Mol. Pharmacol. 1995;47:965–976. [PubMed] [Google Scholar]
- 14.Hamel E, Lin CM. Arch. Biochem. Biophys. 1981;209:29–40. doi: 10.1016/0003-9861(81)90253-8. [DOI] [PubMed] [Google Scholar]
- 15.Grover S, Hamel E. Eur. J. Biochem. 1981;222:163–172. doi: 10.1111/j.1432-1033.1994.tb18854.x. [DOI] [PubMed] [Google Scholar]
- 16.Mandelbaum-Shavit F, Wolpert-DeFilippes MK, Johns DG. Biochem. Biophys. Res. Commun. 1976;72:47–54. doi: 10.1016/0006-291x(76)90958-x. [DOI] [PubMed] [Google Scholar]
- 17.Little M, Ludueña RF. EMBO J. 1985;4:51–56. doi: 10.1002/j.1460-2075.1985.tb02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai R, Pettit GR, Hamel E. J. Biol. Chem. 1990;265:17141–17149. [PubMed] [Google Scholar]
- 19.Bhattacharyya B, Wolff J. FEBS Lett. 1977;75:159–162. doi: 10.1016/0014-5793(77)80075-6. [DOI] [PubMed] [Google Scholar]
- 20.Díaz JF, Menéndez M, Andreu JM. Biochemistry. 1993;32:10067–10077. doi: 10.1021/bi00089a023. [DOI] [PubMed] [Google Scholar]
- 21.Hamel E, Sackett DL, Vourloumis D, Nicolaou KC. Biochemistry. 1999;38:5490–5498. doi: 10.1021/bi983023n. [DOI] [PubMed] [Google Scholar]
- 22.Parness J, Horwitz SB. J. Cell Biol. 1981;91:479–487. doi: 10.1083/jcb.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takoudju M, Wright M, Chenu J, Guéritte-Voegelein F, Guénard D. FEBS Lett. 1988;227:96–98. doi: 10.1016/0014-5793(88)80875-5. [DOI] [PubMed] [Google Scholar]
- 24.Takoudju M, Wright M, Chenu J, Guéritte-Voegelein F, Guénard D. FEBS Lett. 1988;234:177–180. doi: 10.1016/0014-5793(88)81328-0. [DOI] [PubMed] [Google Scholar]
- 25.Dabydeen DA, Florence GJ, Paterson I, Hamel E. Cancer Chemother. Pharmacol. 2004;53:397–403. doi: 10.1007/s00280-003-0755-0. [DOI] [PubMed] [Google Scholar]
- 26.Kumar N. J. Biol. Chem. 1981;256:10435–10441. [PubMed] [Google Scholar]
- 27.Nogales E, Wolf SG, Downing KH. Nature (London) 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 28.Ravelli RBG, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M. Nature (London) 2004;428:198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 29.Gigant B, Wang C C, Ravelli RBG, Roussi F, Steinmetz MO, Curmi PA, Sobel A, Knossow M. Nature (London) 2005;435:519–522. doi: 10.1038/nature03566. [DOI] [PubMed] [Google Scholar]