Figure 1. Potential targets for ketamine and similar agents induce rapid and sustained antidepressant effects.
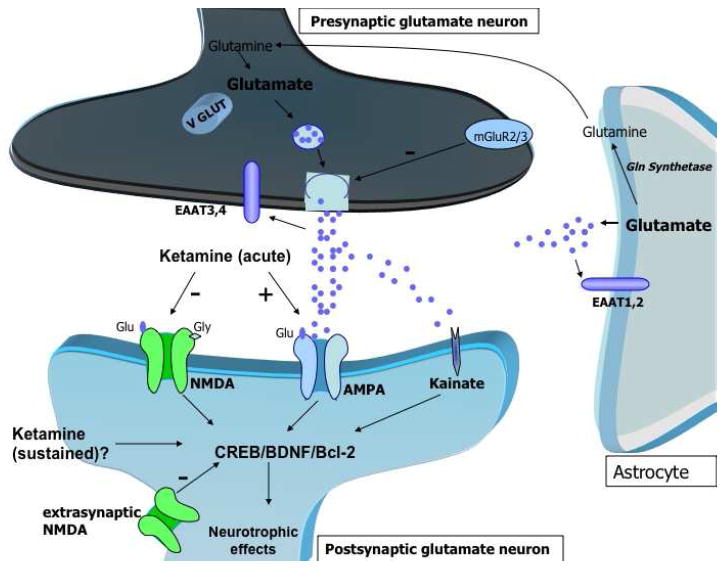
Glutamate is packaged into presynaptic vesicles by the vesicular glutamate transporters (VGLUTs), which critically modulate glutamate concentration in the synaptic vesicles and its consequent release in the synaptic cleft. Also, presynaptic group II mGluR modulation controls glutamate accumulation in the synaptic vesicles. Glutamate clearance from the extracellular space occurs through the high-affinity EAATs presented in the glia and presynaptic neuron. The EAATs play a key role in maintaining adequate neuronal function by reducing potentially toxic extracellular glutamate levels. Notably, ketamine’s rapid antidepressant effects have been shown to be modulated by AMPA relative to NMDA throughput. Excessive glutamate also stimulates the extrasynaptic NMDA receptors, which antagonizes the activation of neurotrophic cascades. The potential sustained (sub-acute) antidepressant effects of ketamine are hypothesized to be mediated by increases in CREB and BDNF expression, as well as the anti-apoptotic protein Bcl-2.
AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid); NMDA (N-methyl-D-aspartate); VGLUTs (vesicular Glu transporters); metabotropic Glu receptors (mGluRs); EAAT (excitatory amino-acid transporters); BDNF (brain-derived neurotrophic factor); CREB (cAMP response element binding); B-cell lymphoma 2 (Bcl-2)
