Summary
Cranial endothermy evolved independently in lamnid sharks, billfishes and tunas, and is thought to minimize the effects of ambient temperature change on both vision and neural function during deep dives. The opah, Lampris guttatus, is a large epipelagic–mesopelagic predator that makes repeated dives into cool waters to forage. To determine if L. guttatus exhibits cranial endothermy, we measured cranial temperatures in live, decked fish and identified potential sources of heat and mechanisms to conserve heat. In 40 opah (95.1±7.6 cm fork length), the temperature of the tissue behind the eye was elevated by a mean (±s.e.m.) of 2.1±0.3°C and a maximum of 6.3°C above myotomal muscle temperature (Tm), used as a proxy for ambient temperature. Cranial temperature varied significantly with Tm and temperature elevation was greater at lower Tm. The proximal region of the paired lateral rectus extraocular muscle appears to be the primary source of heat. This muscle is the largest extraocular muscle, is adjacent to the optic nerve and brain and is separated from the brain only by a thin layer of bone. The proximal lateral rectus muscle is darker red in color and has a higher citrate synthase activity, indicating a higher capacity for aerobic heat production, than all other extraocular muscles. Furthermore, this muscle has a layer of fat insulating it from the gill cavity and is perfused by a network of arteries and veins that forms a putative counter-current heat exchanger. Taken together, these results support the hypothesis that the opah can maintain elevated cranial temperatures.
Keywords: adipose tissue, citrate synthase, counter-current heat exchange, cranial endothermy, extraocular muscle, Lampris guttatus, lateral rectus, magnetic resonance imaging, moonfish, opah, regional endothermy, retia, superior rectus, temperature
INTRODUCTION
Several fish lineages have independently evolved regional endothermy, in which vascular counter-current heat exchangers conserve metabolically derived heat, allowing the temperature of the slow-oxidative locomotor muscle, the viscera, or the cranial region to be elevated significantly above ambient water temperature (Carey et al., 1971; Carey et al., 1985; Block and Finnerty, 1994; Dickson and Graham, 2004). Cranial endothermy, the ability to maintain elevated eye and/or brain temperatures, has evolved by convergence in lamnid sharks (Family Lamnidae), billfishes (Families Xiphiidae and Istiophoridae), tunas (Family Scombridae) and possibly in the butterfly mackerel (Family Scombridae) making cranial endothermy the most widespread form of regional endothermy in fishes (Stevens and Fry, 1971; Linthicum and Carey, 1972; Carey, 1982; Schaefer, 1984; Block and Carey, 1985; Schaefer, 1985; Block, 1991; Block et al., 1993; Sepulveda et al., 2007). Most of these fish species can migrate long distances, make extensive vertical excursions within the water column and experience a wide range of rapidly changing seawater temperatures (Carey et al., 1971; Carey and Lawson, 1973; Carey et al., 1981; Carey, 1990; Josse et al., 1998; Dagorn et al., 2000; Schaefer and Fuller, 2002; Musyl et al., 2003; Brill et al., 2005; Schaefer et al., 2007). In addition, these species are pelagic predators, have large eyes and rely on vision to seek and capture active prey. It has been hypothesized that cranial endothermy improves the ability to forage in cold, dark waters in two ways. First, it buffers the central nervous system from rapid changes in ambient temperature (Carey, 1982; Block, 1987b; Carey, 1990; Block et al., 1993; Block and Finnerty, 1994). Second, maintaining elevated cranial temperatures improves the ability to resolve intermittent visual stimuli by an increase in the flicker fusion frequency (Fritsches et al., 2005), enhancing the ability to track fast moving prey. Cranial endothermy probably evolved to allow fishes to forage successfully in deep cool waters and thus expand their thermal niche and forage base (Block et al., 1993).
Cranial endothermy requires a metabolic heat source and a mechanism to retain that heat and is achieved by different means in the different fish groups. The heat source in billfishes and the butterfly mackerel consists of specialized heater tissue derived from the superior rectus and the lateral rectus extraocular muscles, respectively (Block, 1987a; Finnerty and Block, 1992). These heater tissues are composed of modified muscle cells that lack contractile filaments, are densely packed with mitochondria and have an extensive sarcoplasmic reticulum and a high oxidative capacity (Block, 1991; Tullis et al., 1991). Heat production in heater cells is associated with the ATP-dependent cycling of Ca2+ across the sarcoplasmic reticulum membrane (Block, 1987a; Block, 1991). The only other species known to have modified extraocular muscle is the slender tuna (Allothunnus fallai), the most basal tuna, in which portions of the superior, inferior, medial and lateral rectus muscles are fused to form a putative heater tissue located ventral to the brain (Sepulveda et al., 2007). In the other tuna species, there is no evidence of modified extraocular muscle (Block et al., 1982; Block, 1990; Sepulveda et al., 2007), and contraction of the extraocular muscles (Block and Finnerty, 1994) or active metabolism within the brain (Block, 1987b; Block et al., 1982) have been proposed as possible sources of heat for cranial endothermy in those species. In lamnid sharks, heat produced within the slow-oxidative myotomal muscle is transferred to the cranial area via the unique red muscle vein and the myelonal vein (Block and Carey, 1985; Carey et al., 1985; Wolf et al., 1988; Alexander, 1998; Tubbesing and Block, 2000). In addition, contraction of extraocular muscles may also contribute to heat production in these sharks (Wolf et al., 1988; Alexander, 1998).
Regardless of the heat source, the high heat capacity of water and use of gills for gas exchange necessitate mechanisms that reduce both conductive and convective heat loss to the environment and that conserve heat within the cranial region. To reduce conduction, the eyes and brain of billfishes and tunas, but not lamnid sharks, are insulated by a layer of adipose tissue (Carey, 1982; Wolf et al., 1988; Block, 1990; Fritsches et al., 2005; Sepulveda et al., 2007). To reduce convection of heat to the gills, vascular counter-current heat exchangers (retia mirabilia) perfuse the cranial region in all fish species with cranial endothermy but retia morphology differs among the fish groups. Billfishes and tunas have paired carotid retia composed of arteries branching primarily from the carotid arteries and veins that eventually empty into the anterior cardinal vein (Linthicum and Carey, 1972; Carey, 1982; Block, 1986; Block, 1990; Sepulveda et al., 2007). In the butterfly mackerel, the putative counter-current heat exchanger perfusing the lateral rectus muscle is more posterior, branching from the lateral dorsal aorta (Block, 1991). The lamnid sharks have paired orbital retia, each made of an arterial plexus branching from the highly coiled pseudobranchial and efferent hyoidean arteries surrounded by warm blood within the orbital venous sinus (Block and Carey, 1985; Carey et al., 1985; Alexander, 1998; Tubbesing and Block, 2000). The red muscle vein supplies warm blood to the orbital sinus via the myelonal vein (Wolf et al., 1988; Tubbesing and Block, 2000).
These heat production and conservation mechanisms allow cranial temperatures to be elevated above ambient water temperature by an amount that varies by species and with water temperature. Cranial temperatures measured in decked fishes representing the different taxonomic groups ranged from 0.4 to 15.5°C above water temperature (Stevens and Fry, 1971; Linthicum and Carey, 1972; Carey, 1982; Block and Carey, 1985; Block, 1987b; Carey, 1990; Block, 1991; Sepulveda et al., 2007). The highest temperatures have been observed in giant Atlantic bluefin, Thunnus thynnus (Linthicum and Carey, 1972) and in swordfish, Xiphias gladius, in which cranial temperatures elevated 13°C above water temperature were recorded during deep dives using acoustic telemetry (Carey, 1990). Unfortunately, measurements of cranial temperature in free-swimming fishes are scarce.
Previous studies have noted that the opah or moonfish [Lampris guttatus (Brünnich 1788) Order Lampridiformes] possesses vascular modifications within the cranial region that are suggestive of endothermy (Block, 1986; Block, 1987b). L. guttatus is a large epipelagic–mesopelagic predator, reaching sizes of 144 kg, with large eyes (Polovina et al., 2008). It shares certain anatomical adaptations with the endothermic billfishes, tunas and lamnid sharks, including a large muscular heart, well-developed pyloric ceca and a large relative locomotor muscle mass (Rosenblatt and Johnson, 1976) (H.D., unpublished). Based on catch data, the geographical range of opah extends from temperate through to tropical waters in both hemispheres (Gon, 1990). A recent study of 11 opah tagged with pop-up archival tags off of Hawaii showed that opah consistently move vertically within the depth range of 50–400 m, encountering water temperatures ranging from 8 to 22°C and reach a maximum depth of 736 m (Polovina et al., 2008). Stomach content data show that opah feed on squid and fishes (Palmer, 1986; Polovina et al., 2008). Thus, like the other cranial endotherms, L. guttatus experiences a broad range of ambient temperatures, forages on fast moving prey in cool deep waters and would benefit from regional endothermy.
The purpose of this study was to test for cranial endothermy in the opah. In order to establish cranial endothermy, one must measure elevated cranial temperatures and identify both a heat source and a mechanism to conserve heat in the cranial region. In this study, we measured elevated cranial temperatures in live opah caught by commercial long-liners and used dissections, light and electron microscopy, biochemical analyses and magnetic resonance imaging (MRI) to identify and describe the potential heat sources and heat retention mechanisms in L. guttatus.
MATERIALS AND METHODS
Fish collection
Opah, Lampris guttatus (Brünnich 1788), were caught by long-line during commercial fishing operations targeting tunas in the North Pacific Ocean. Temperature measurements, extraocular muscle samples fixed for electron microscopy and muscle samples frozen at –80°C for enzymatic analysis were obtained from opah caught offshore of the main Hawaiian Islands. These individuals ranged in size from 74.8 to 114.6 cm fork length (FL). Opah heads used in dissections and MRI imaging and from which tissue samples were removed for light microscopy and enzymatic analysis, were obtained from Chesapeake Fish Company, San Diego, CA, USA; the lengths and masses of these opah were not available. These fish were caught off of the southern California coast and kept on ice prior to being processed. Extraocular muscle samples for enzymatic analysis were removed from some of these opah heads and frozen at –80°C; other opah heads were frozen at –20°C until they were thawed for dissections of the cranial and orbital region, MRI imaging or removal of tissue samples for light microscopy. Because the fish were obtained opportunistically from commercial fishers, it was not always possible to control conditions of sample collection.
Measurement of cranial temperatures
A total of 121 opah were caught between 2002 and 2006 during commercial long-line fishing operations in the central Pacific Ocean off of the main Hawaiian Islands between 18–30 deg. N and 136–153 deg. W. Immediately after fish were brought aboard and before the fish were processed by the fishermen, individuals were placed on their right side, their condition was noted (dead or alive), FL was measured to the nearest mm, they were sexed and tissue temperatures were recorded. A waterproof, heavy-duty K-type thermocouple thermometer (HI 9063, Hanna Instruments, Póvoa de Varzim, Portugal) and a 12 cm thermocouple probe were used to obtain temperatures within the cranial region near the brain and in the deep, fast-twitch, glycolytic myotomal muscle tissue anterior to the pectoral fin. Because the temperature at catch depth is unknown, myotomal muscle temperature was used as a proxy for ambient temperature, as has been done in previous studies (Carey et al., 1971; Linthicum and Carey, 1973; Bernal and Sepulveda, 2005). There is no evidence that temperatures of the myotomal or pectoral fin locomotor muscles can be elevated significantly above ambient temperature in the opah (Carey et al., 1971; Rosenblatt and Johnson, 1976). The magnitude of cranial temperature elevation or cranial thermal excess (Tx) was calculated for each individual as the difference between maximal cranial temperature and myotomal muscle temperature, as in previous studies (Carey et al., 1971; Linthicum and Carey, 1973; Bernal and Sepulveda, 2005).
Identification and characterization of a heat source in the opah
Gross anatomy of the cranial region and relative size of the extraocular muscles
Ten opah heads were dissected to identify cranial tissues that may contribute to heat production or heat retention. Characteristics used during dissections to identify potential heat sources for cranial endothermy included position within the cranial cavity (near the brain and optic nerve), relative size and the redness of the tissue. How red in color a muscle tissue appears results from the presence of myoglobin and blood and serves as a relative indicator of tissue aerobic capacity and associated heat production.
The masses of each extraocular muscle and the eyeball were measured in eyes dissected from seven of the opah heads. The whole eyeball and the attached extraocular muscles were removed and all visible fat was dissected away. Each extraocular muscle was separated from the eye and weighed individually (to the nearest 0.01 g), and the mass of each extraocular muscle was expressed as a percentage of total eye mass (eyeball with optic nerve and sum of all muscles). In addition, the masses of all six extraocular muscles were summed and total extraocular muscle mass, expressed as a percentage of total eye mass, was calculated for the opah and compared with values obtained in the same manner from an active ectothermic teleost species, the chub mackerel (Scomber japonicus, Family Scombridae).
Extraocular muscle histology
Light microscopy and transmission electron microscopy (TEM) were used to determine if any of the six extraocular muscles of the opah are modified to form specialized heater tissue as in billfishes, butterfly mackerel and slender tuna. Muscle transverse sections were removed from proximal (near the origin of the muscle on the skull), middle and distal (near the insertion on the eyeball) positions along the lateral rectus extraocular muscle, from the middle and proximal portions of the medial rectus and superior rectus extraocular muscles and from the middle portion of the three other extraocular muscles. These samples were fixed in 10% phosphate-buffered formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin (Harris Histology Services, Tustin, CA, USA).
Tissue samples used for TEM analysis were collected from three opah captured by long-line fishing operations between Oregon and Hawaii. Samples from all six extraocular muscles (approximately 12×12 mm) were removed immediately after each fish was euthanized and were fixed in 2% glutaraldehyde and 2% formaldehyde in 0.1 mol l–1 cacodylate buffer, pH 7.4 at 4°C for 1.5–2.0 h. Specimens were then stored cold in approximately 0.5% glutaraldehyde and 0.5% formaldehyde in 0.1 mol l–1 cacodylate buffer for four weeks until they were sent to California State University Fullerton (CSUF). From the surface of each 12×12 mm sample, small tissue samples (1×3 mm) were removed and washed three times in buffer (0.1 mol l–1 sodium phosphate buffer, pH 7.2 at room temperature) for 30 min. The tissues were fixed in 2% osmium tetroxide in buffer, dehydrated in a graded ethanol series and embedded in epoxy blocks with the long axis of the muscle fibers parallel to the long axis of the block. Ultrathin sections (80 nm thick) were cut using an ultramicrotome and a diamond knife, mounted onto copper TEM grids, stained with 3% uranyl acetate and 1.5% lead citrate and examined with a Hitachi H7000 TEM at CSUF.
Extraocular muscle aerobic heat production capacity
Samples of each extraocular muscle were used to quantify the activity of the citric acid cycle enzyme citrate synthase (CS) as an index of tissue mitochondrial density and aerobic heat production capacity. For CS assays, muscle samples were homogenized on ice with a ground-glass homogenizer in 2 mmol l–1 ethylene diamine tetra-acetic acid, 80 mmol l–1 imidazole buffer, pH 6.6 at 20°C and centrifuged in a high-speed, refrigerated centrifuge at 12,000× g for 10 min. The supernatant containing soluble enzymes was removed for measurements of CS activity using a Hewlett-Packard 8452A diode-array spectrophotometer (Palo Alto, CA, USA) and assay procedures used in previous studies of other fishes (Dickson et al., 1993; Dickson, 1996). Assays were run at 20°C in a final volume of 2.0 ml containing 0.5 mmol l–1 oxaloacetate, 0.10 mmol l–1 acetyl Co-enzymeA, 0.10 mmol l–1 5,5′-dithiobis(2-nitrobenzoic acid), 2.0 mmol l–1 MgCl2 and 80 mmol l–1 Tris buffer, pH 8.0 at 20°C. Enzyme assays were carried out under saturating substrate conditions, as determined in preliminary studies. Enzyme activities are expressed as micromoles of substrate converted to product min–1 (international units) per gram wet mass of muscle tissue (units g–1).
From five opah captured on long-line off of Hawaii, the left eye along with attached extraocular muscles was removed, placed into liquid nitrogen immediately after capture, sent to CSUF on dry ice and stored at –80°C for up to two months. Each eye was thawed, and small samples from each extraocular muscle were removed from a position approximately one-third along the length of the muscle from its insertion on the eyeball for CS assays. However, these eyes did not include the proximal portions of the extraocular muscles. Samples of the proximal and distal regions of the lateral rectus muscle, as well as the pectoral fin and myotomal muscles, of one opah were collected immediately after the fish was captured by long-line and euthanized, frozen immediately in liquid nitrogen and stored at –80°C. An additional five opah were chilled on ice for up to six days on a Chesapeake Fish Company commercial fishing vessel, after which the heads were brought to CSUF where extraocular muscle samples were removed and stored at –80°C for up to two weeks. From these five fish, samples for CS assays were taken from each extraocular muscle at a position approximately one-third along the length from its insertion onto the eyeball, from the proximal and distal portions of the lateral rectus muscle and from the proximal portion of the superior rectus muscle.
Normally, enzyme activities are quantified in tissue samples that are frozen immediately and stored at –80°C to minimize protein degradation. However, because all other attempts to obtain –80°C frozen extraocular muscle samples from opah were unsuccessful, it was necessary to use chilled muscle samples to obtain enough CS activity measurements, particularly for the proximal region of the lateral rectus muscle. To test the validity of using those samples, both lateral rectus extraocular muscles were removed from five chub mackerel immediately after the fish were caught by hook and line and euthanized. One muscle was immediately frozen at –80°C whereas the other was chilled on ice for six days before being frozen at –80°C. These two sets of samples were handled in the same way that the different opah extraocular muscle samples had been handled prior to running CS assays. The CS activities of the two sets of lateral rectus muscle samples from the chub mackerel (28.0±5.7 and 25.0±3.9 units g–1, means±s.d., for frozen and chilled samples, respectively) did not differ significantly (paired t-test; P>0.05). Likewise, in the opah, the mean CS activities for each extraocular muscle for which both chilled and frozen samples were obtained did not differ significantly (two-sample t-test; P>0.05). Therefore, the chilled and frozen data sets for opah were combined.
Identification and characterization of heat retention mechanisms in the opah
Possible mechanisms to retain heat in the cranial and orbital region were investigated in gross dissections of opah heads. We looked for adipose tissue that would insulate the cranial and orbital region and reduce conduction to the surrounding seawater. In addition, a hypothesized counter-current heat exchange system, which perfuses several of the extraocular muscles and would reduce convective heat loss, was identified. The origin of the blood vessels that form this putative heat exchanger were traced in dissections. Characteristics used to identify the putative counter-current heat exchanger included numerous arteries and veins adjacent to one another with a high surface area of contact. To confirm that the putative counter-current heat exchanger was composed of adjacent arteries and veins, samples were fixed in 10% phosphate-buffered formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin at Harris Histology. Histological sections were examined by light microscopy, and arteries and veins were distinguished by the thickness of the blood vessel wall; one sample was also stained for elastin and one with trichome stain to confirm the identification of the blood vessels. In addition, samples of the fat found in dissections to insulate the cranial region were fixed in phosphate-buffered formalin and sent to Harris Histology to be embedded in paraffin, sectioned and stained with hematoxylin and eosin.
Three dimensional visualization using magnetic resonance imaging
To visualize and document the 3 dimensional arrangement of the tissues within the cranial region, one opah head was studied using MRI. MRI data were acquired over a portion of the opah head on a 3.0T SIGNA General Electric (Milwaukee, WI, USA) clinical scanner and a standard quadrature head radio frequency receive coil at the Keck Center for Functional Magnetic Resonance Imaging, University of California San Diego. The following MRI parameters were used: transverse orientation; T1-weighted 3-D fast spoiled gradient recalled echo acquisition; flip angle of 10 deg.; echo time TE=3.288 ms; repetition time TR=7.864 ms; full k-space acquisition; 20 cm field of view; in plane image matrix of 256×256; 124 slices with 1.0 mm slice thickness; and 31.25 kHz bandwidth. Data were processed using Amira software (Mercury Computer Systems, Chelmsford, MA, USA) to determine the shape and relative geometry of the extraocular muscles, brain, skull and surrounding adipose tissue. Segmentation analysis differentiated the various tissue volumes on the basis of their intrinsic T1-weighted image intensities. The segmented volumes allowed both quantitative measures of tissue volume and 3 dimensional visualization of the relative positions of the cranial tissues.
Statistical analyses
Minitab (v. 12; Minitab, State College, PA, USA) was used for all statistical analyses. Data were examined for normality (Kolmogorov–Smirnov test) and homogeneity of variance (Bartlett's and Levene's tests) and data that did not meet the assumption of homogeneity of variance were log- or square root-transformed. To test for significant differences between cranial temperature and myotomal muscle temperature, we used a paired t-test. To test for significant relationships between cranial temperature and both fish FL and muscle temperature, we used Pearson product–moment correlation coefficients. General Linear Model Analysis of Variance (ANOVA) was used to test for differences among the extraocular muscle samples in CS activity or relative mass. If a significant difference was found, Tukey's pairwise comparison test was used to identify significant differences among the individual muscles. To test for differences between opah and chub mackerel in extraocular muscle mass as a percentage of total eye mass, a two-sample t-test was used. A significance level of α=0.05 was used in all statistical analyses. Unless stated otherwise, all values are means±1 s.d.
RESULTS
Temperature measurements
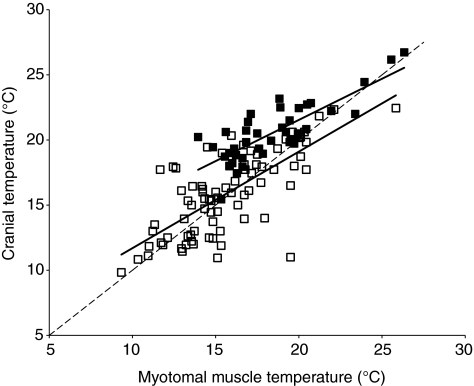
Of 121 opah collected by long-line gear off of Hawaii, 81 (66%; 43 male and 38 female) were dead and 40 (34%; 13 male and 27 female) were alive when decked. The live opah ranged in FL from 74.8 to 108.0 cm (95.1±7.6 cm) whereas the dead opah ranged from 79.8 to 114.6 cm FL (99.8±7.8 cm). Sea surface temperature data when each opah was captured were obtained from the Geostationary Operational Environmental Satellite Program and ranged from 18.9 to 26.6°C with a mean of 23.2±1.8°C. In the live opah, the temperature of the cranial region, specifically the tissue behind the eyes, was significantly greater than the white myotomal muscle temperature, a proxy for ambient temperature (paired t-test, P<0.001). The mean cranial temperature of the live opah was 20.6°C and the mean white myotomal muscle temperature was 18.5°C. Cranial temperature varied with muscle temperature and the difference between cranial temperature and muscle temperature was greater at lower temperatures than at higher temperatures (Fig. 1). The mean (±s.e.m.) cranial thermal excess (Tx) for these fish was 2.1±0.3°C, with a maximum Tx of 6.3°C. In the opah that were brought aboard the long-line vessel dead, there was no statistically significant difference between the temperature of the cranial region and the white myotomal muscle temperature (paired t-test, P=0.19); the Tx for those fish ranged from –8.5 to +6.1°C with a mean (±s.e.m.) of 0.2±0.3°C (Fig. 1). The relationship between cranial temperature and muscle temperature in the dead opah did not differ significantly from the isothermal line (Fig. 1). Tx did not vary significantly with fish FL in either the live or dead opah (data not shown; R=–0.29 for live opah and R=–0.20 for dead opah; P>0.05).
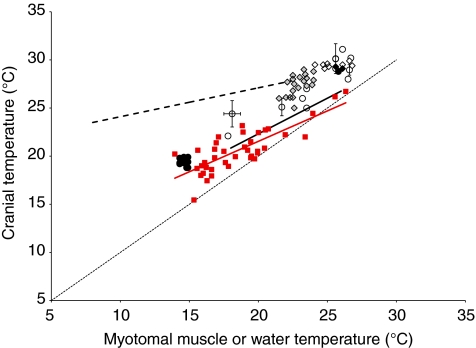
Fig. 1.
Cranial temperature as a function of deep, fast-twitch, glycolytic myotomal muscle temperature, used as a proxy for ambient water temperature, in 40 opah that were alive when decked by long-line gear (solid squares) and in 81 dead opah (open squares). The best-fit regression for the live opah is cranial temperature=0.632×muscle temperature+8.90 (R=0.81, P<0.05) and for the dead opah is cranial temperature=0.741×muscle temperature+4.29 (R=0.73, P<0.05). The broken line represents isothermal conditions (cranial temperature=muscle temperature).
Identification and characterization of potential heat sources in the opah
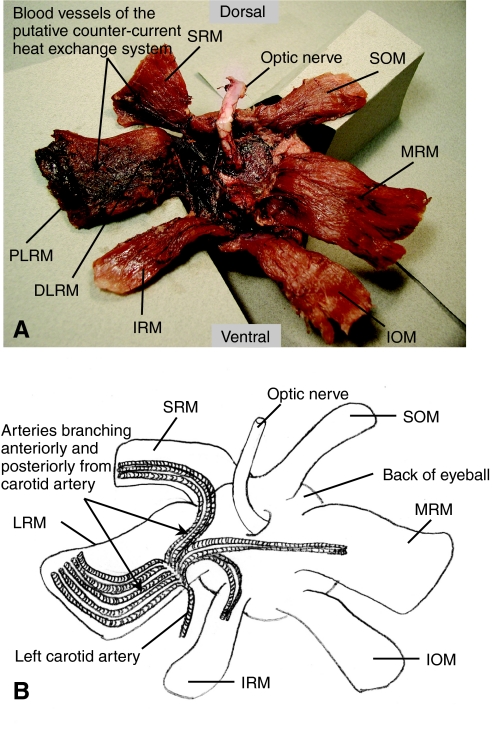
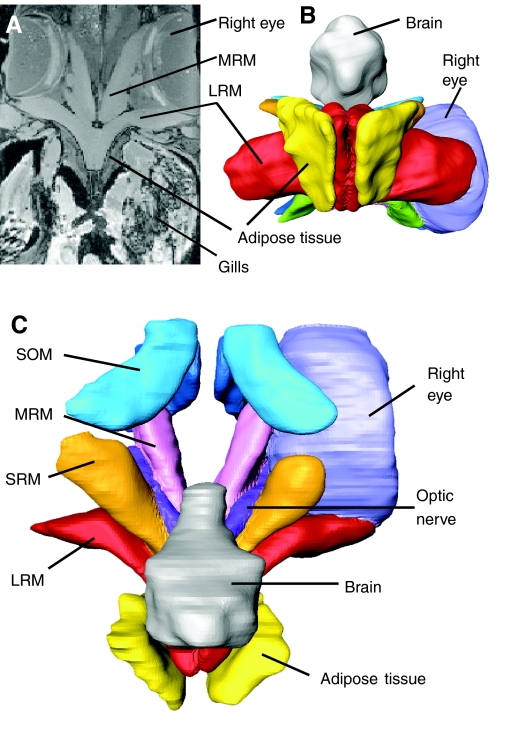
Based on dissections and MRI imaging of the cranial and orbital region, the proximal region of the lateral rectus muscle (PLRM) was identified as the most likely heat source for cranial endothermy in the opah. The PLRM is positioned adjacent to the brain, is composed of muscle fibers that are darker red in color than those of the distal region of the lateral rectus muscle (DLRM) and the other extraocular muscles (Fig. 2A) and is perfused by a putative counter-current heat exchanger (described below). The lateral rectus extraocular muscle originates on the anterior side of the basioccipital bone within the posterior portion of the myodome and inserts on the eyeball opposite the medial rectus muscle. The superior rectus extraocular muscle also originates on the anterior side of the basioccipital bone and inserts dorsally on the eyeball between the lateral rectus and superior oblique extraocular muscles. The other extraocular muscles – the medial rectus (MRM), superior rectus (SRM), inferior rectus (IRM), superior oblique (SOM) and inferior oblique (IOM) – are composed of muscle fibers that are less red in color than the PLRM (Fig. 2A). The MRM and IRM originate on the basisphenoid bone whereas the SOM and IOM originate on the pterosphenoid bone. Dissections and MRI imaging (Fig. 3) also revealed that the PLRM is adjacent to the brain and well insulated by fat (approximately 2.5 cm thick as determined from MRI), which is made up of white adipose tissue, as documented by light microscopy (not shown). The thick mass of fat overlies the thin opisthotic (intercalary) bone that overlies the PLRM and lies between the PLRM and the gill cavity (Fig. 3). The PLRM is separated from the brain by a thin layer of bone associated with the braincase.
Fig. 2.
(A) Photograph of the six extraocular muscles in opah, viewed from the back of the left eye. Proximal and distal regions of the lateral rectus (PLRM and DLRM), medial rectus (MRM), superior rectus (SRM), inferior rectus (IRM), superior oblique (SOM) and inferior oblique (IOM) are labeled. (B) A schematic representation of the arterial circulation from the carotid artery to the lateral rectus (LRM), SRM, MRM and IRM, based on gross dissections. Illustration is based on Fig. 2A.
Fig. 3.
Magnetic resonance image (MRI) of the cranial region of the opah and resulting 3-D reconstructions using image segmentation. (A) MRI coronal section, approximately midway through eyes showing the position of the extraocular muscles relative to the eye, skull, brain, adipose tissue and gill cavity. (B) Transverse view and (C) coronal view 3-D models created from segmentation of the MRI data showing the relative positions of the extraocular muscles, insulating fat, and brain. In the 3-D models, only the right eye is shown. Lateral rectus (LRM), medial rectus (MRM), superior oblique (SOM) and superior rectus (SRM) extraocular muscles are labeled.
Relative size of the extraocular muscles
The mean relative extraocular muscle mass as a percentage of total eye mass (eyeball and extraocular muscle mass) in the opah (40.1±2.3%, N=7) was significantly higher than that in the ectothermic chub mackerel (12.9±2.1%, N=5) (t-test, P=0.0001). In opah and chub mackerel, the LRM is significantly larger (mass as a percentage of total eye mass) than all other extraocular muscles (ANOVA, P<0.05), and the MRM is significantly larger than the SRM, IRM, SOM and IOM (ANOVA, P<0.05) (Table 1). The relative mass of each individual muscle is significantly greater in opah than in chub mackerel (two-sample t-tests, P<0.05).
Table 1.
Mean (±s.d.) relative mass of each extraocular muscle (muscle mass as percentage of total eye mass) in the opah, Lampris guttatus (N=7) and the chub mackerel, Scomber japonicus (N=5)
| Extraocular muscle | Lampris guttatus (%) | Scomber japonicus (%) |
|---|---|---|
| Lateral rectus | 11.70±1.04 | 4.81±0.83 |
| Superior rectus | 4.33±0.58 | 1.64±0.49 |
| Inferior rectus | 4.27±0.66 | 1.51±0.55 |
| Medial rectus | 10.27±0.98 | 2.68±0.68 |
| Superior oblique | 4.56±0.62 | 1.56±0.22 |
| Inferior oblique | 4.92±0.28 | 1.70±0.71 |
In both species, the lateral rectus muscle is significantly larger than all other extraocular muscles (ANOVA, P<0.05) and the medial rectus is significantly larger than the other four extraocular muscles (ANOVA, P<0.05). The mean relative mass of each extraocular muscle is significantly greater in opah than in chub mackerel (two-sample t-test, P<0.05)
Extraocular muscle histology
Microscopic inspection of the extraocular muscles in the opah provided no evidence that these muscles are modified for heat production like the specialized heater tissue of billfishes, butterfly mackerel and slender tuna. When examined by light microscopy, longitudinally sectioned muscle fibers within each of the extraocular muscles were striated. Transmission electron micrographs of all opah extraocular muscles, including the PLRM (Fig. 4), indicate that these muscles are made up of normal striated muscle fibers containing myofibrils.
Fig. 4.
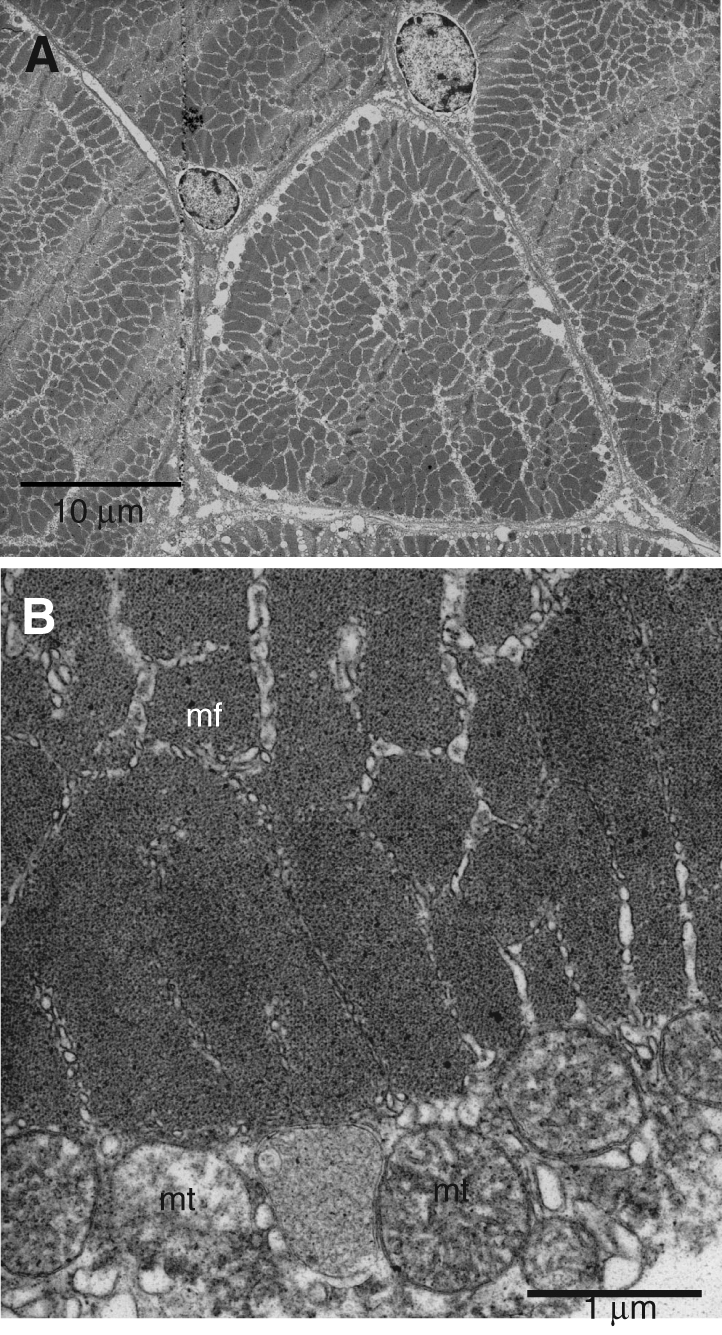
Transmission electron micrographs (TEMs) of transverse sections of the proximal portion of the lateral rectus extraocular muscle. (A) An entire extraocular muscle fiber filled with myofibrils. n=nucleus of adjacent muscle fibers. (B) Higher magnification of a portion of a proximal lateral rectus muscle fiber showing the regular array of thick and thin filaments within the myofibrils (mf). mt=subsarcolemmal mitochondria.
Extraocular muscle aerobic heat production capacity
The PLRM had the highest mean CS activity relative to all other extraocular muscles (Table 2). The CS activity in the PLRM was 36.1±18.6 units g–1 (N=6), which was significantly higher than in the DLRM, SRM, IRM, IOM and SOM (ANOVA, P<0.05). The CS activity of the PLRM did not differ significantly from that of the MRM (ANOVA, P>0.05) and CS activity in the MRM was significantly higher than in the IOM (ANOVA, P<0.05).
Table 2.
Mean (±s.d.) citrate synthase (CS) activity [μmol of substrate converted to product min–1 (international units) g–1 wet mass of tissue] at 20°C in opah extraocular muscles
| N | Extraocular muscle | Citrate synthase activity (units g–1) |
|---|---|---|
| 6 | Lateral rectus, proximal portion | 36.13±18.6 |
| 7 | Lateral rectus, distal portion | 3.65±1.62 |
| 9 | Superior rectus | 6.54±2.05 |
| 9 | Inferior rectus | 5.42±3.20 |
| 10 | Medial rectus | 13.13±3.85 |
| 2 | Medial rectus, proximal portion | 12.46 |
| 8 | Superior oblique | 7.33±5.34 |
| 8 | Inferior oblique | 3.22±1.40 |
CS activity is significantly higher in the proximal portion of the lateral rectus muscle (LRM) than in the distal portion of the LRM, the superior rectus, inferior rectus, superior oblique and inferior oblique muscles (ANOVA, P<0.05). In addition, the medial rectus muscle has a significantly higher CS activity than the inferior oblique muscle (ANOVA, P<0.05)
In one opah, the CS activity at 20°C of slow-twitch myotomal muscle (4.89 units g–1), fast-twitch myotomal muscle (1.59 units g–1) and red and white regions of the pectoral fin muscle (5.10 and 1.57 units g–1, respectively) were also measured. In this individual, PLRM CS activity was 27.2 units g–1, which is more than five times the highest CS activity measured in its locomotor muscles.
Identification and characterization of potential heat retention mechanisms in the opah
The proximal portion of the lateral rectus extraocular muscle in opah is perfused by a putative counter-current heat exchange system composed of numerous parallel blood vessels that were observed on the medial surface of the LRM in dissections (Fig. 2B). The proximal portion of the superior rectus extraocular muscle (PSRM) is also perfused by a putative counter-current heat exchange system that is less extensive than that of the PLRM. The arterial vessels of both systems originate from the carotid arteries. The efferent branchial arteries carry cool oxygenated blood from the gills and empty into the dorsal aorta that branches to form the paired dorsal aortas, which continue anteriorly towards the head. The two carotid arteries branch from the right and left dorsal aortas and enter the cranium through ostia (one ostium on each side of the skull) in the thin opisthotic bone. Small arteries branch from each carotid artery – some extend anteriorly and others extend posteriorly (Fig. 2B). The anterior branches of each carotid artery give off a series of small arteries that supply blood to the distal portion of the lateral rectus muscle and to the superior rectus, inferior rectus and medial rectus extraocular muscles of each eye (Fig. 2B). From the posterior branches of the carotid artery, a series of small arteries supply blood to the proximal region of the lateral rectus muscle and form the arterial part of a putative counter-current heat exchanger.
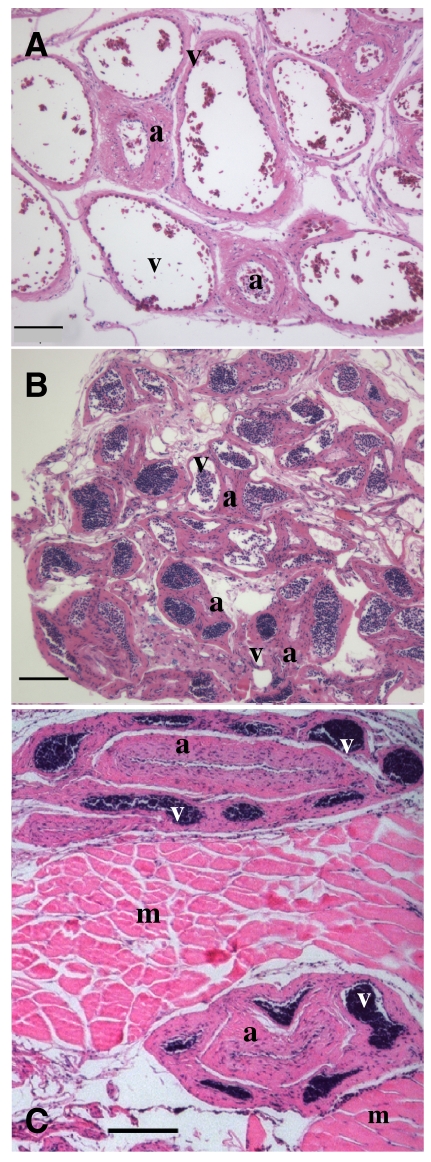
Light microscopic analysis of the parallel blood vessels from the proximal region of the LRM revealed thick-walled arteries surrounded by thin-walled veins (Fig. 5). There is a network of blood vessels along the medial surface of the proximal LRM, with extensive contact between adjacent arteries and veins that would facilitate heat exchange. In one opah of unknown size, this network had maximum dimensions of 9×1 mm and was made up of 86 blood vessels along its length, with 12 blood vessels at its maximum width (Fig. 5B). Within this putative heat-exchanger, arterial outer diameters ranged from 35.0 to 93.8 μm (59.4±10.1 μm, N=64) and vein diameters ranged from 35.6 to 121.2μm (85.9±16.3μm, N=75). The blood vessels making up the putative counter-current heat exchanger to the LRM branch anteriorly towards the insertion of the LRM in groups of arteries and veins that penetrate into the muscle medio-laterally to perfuse the muscle fibers of the LRM. Within the LRM, these groups of arteries and veins are separated by lateral rectus extraocular muscle fibers (Fig. 5C).
Fig. 5.
(A. and B) Light micrographs of transverse sections through the putative counter-current heat exchanger perfusing the proximal portion of the lateral rectus extraocular muscle (PLRM) showing arteries (a) surrounded by veins (v). Inside most blood vessels are darkly stained nucleated red blood cells. In (B) almost the entire width of the heat exchanger located on the medial surface of the PLRM is shown, illustrating the network of adjacent arteries (a) and veins (v). In this opah, the entire rete had a maximum width of 1 mm, with up to 12 adjacent blood vessels and a maximum of 86 adjacent blood vessels along its length of 9 mm. (C) Section of the lateral rectus extraocular muscle (LRM) approximately midway between its origin and insertion, showing the arteries (a), surrounded by veins (v), that penetrate the muscle medio-laterally, separated from other artery–vein groups by muscle fibers (m). These blood vessels are continuous with those that make up the counter-current heat exchanger. Scale bars are 100 μm.
In addition, a second putative counter-current heat exchange system was observed on the medial surface of the proximal region of the SRM. Parallel blood vessels branch from the anterior continuation of each carotid artery to perfuse the SRM (Fig. 2B). This putative counter-current heat exchange system is composed of adjacent arteries and veins along the medial surface of the PSRM but there are fewer blood vessels than observed in the proximal portion of the LRM.
There is no evidence of a counter-current heat exchange system perfusing the MRM or any of the other extraocular muscles. In these muscles, blood vessels are dispersed throughout the extraocular muscles and are not in contact with one another in multiple rows as required for effective counter-current heat exchange.
The blood vessels that make up the putative counter-current heat exchangers perfusing the proximal regions of the LRM and SRM would allow heat from the venous blood leaving these muscles to be transferred to the cool blood in the arteries, thereby reducing convective heat loss from the LRM and the SRM to the gills. In addition, conduction of heat to the surrounding seawater from the back of the eye and the proximal regions of both muscles is reduced by the presence of fat. The PLRM and PSRM are both insulated by a layer of white adipose tissue (approximately 2.5 cm thick) that overlies the thin opisthotic bone, which overlies the PLRM. The MRI clearly illustrates how the fat is positioned between the PLRM and the seawater within the gill cavity (Fig. 3A). In addition, the back of each eyeball is entirely surrounded by an approximately 1 cm thick layer of fat.
DISCUSSION
Taken together, our results support the hypothesis that the opah can maintain elevated cranial temperatures, which would add a completely new order to the list of fishes capable of regional endothermy. We found that cranial temperatures are elevated significantly above ambient temperature when using muscle temperature as a proxy. In addition, potential mechanisms for both heat production and heat retention in the cranial region were identified. Specifically, heat generated by contraction of the lateral and superior rectus extraocular muscles should be conserved within the cranial region by vascular counter-current heat exchangers and insulating adipose tissue, which would enable the opah to elevate cranial temperatures. The mechanisms described in opah are most similar to those evolved in tunas of the genus Thunnus (Linthicum and Carey, 1972).
Cranial temperatures in live opah were elevated by a mean of 2.1°C and by as much as 6.3°C when compared with myotomal muscle temperature (Fig. 1). Although one live opah had a cranial temperature lower than muscle temperature and several individuals were on or near the isothermal line, most had elevated cranial temperatures, with Tx values similar to those of some other fish species known to be cranial endotherms (Fig. 6). It might be possible for the Tx measured in the opah to result from more rapid rates of warming of the cranial region than of the core muscle as fish, which are caught below the thermocline, pass through the warm, mixed layer on their way to the surface. Opah are typically caught at depths of 265±73 m in 13.0±3.5°C water, where the surface temperature is approximately 18–27°C (D.R.H., unpublished). During retrieval of the long-line gear, opah spend approximately 7 min in the upper, mixed layer (D.R.H., unpublished). However, several lines of evidence argue against this possibility. First, the temperature data for live opah differ from that for dead opah. Even though there is some overlap and some dead opah had high Tx values, the live opah overall had significantly elevated cranial temperatures relative to core muscle temperature whereas the dead fish did not (Fig. 1). Second, only one live opah but 30 of the dead opah had Tx values less than 0°C, meaning that muscle temperature was actually greater than cranial temperature. Because opah are laterally compressed, the cranial region and the locomotor muscle in which core temperatures were measured are a similar distance from the body surface and should warm by conduction at comparable rates but the cranial area may warm more slowly due to the presence of insulating fat. Third, if absorption of heat from the environment explains the elevation of cranial temperatures in opah, then one would expect smaller individuals to have warmed faster than larger individuals and, thus, have a larger difference between cranial temperature and core muscle temperature. However, we found no significant effect of FL on cranial temperature or on Tx.
Fig. 6.
Cranial temperature as a function of deep myotomal muscle temperature in the live opah from Fig. 1 (red solid squares and red line). Also plotted are cranial temperature versus ambient temperature data for other fish species known to be cranial endotherms: solid black line, shortfin mako shark (Block and Carey, 1985); broken line, giant (∼200–450 kg) Atlantic bluefin tuna (Linthicum and Carey, 1972); diamonds, billfish species – blue marlin (open), white marlin (black filled) and spearfish (gray filled) (Block, 1991); open circles, tuna species – small Atlantic bluefin, albacore, bigeye, little tunny (Linthicum and Carey, 1972), skipjack (Stevens and Fry, 1971), black skipjack (Schaefer, 1984), frigate tuna (Schaefer, 1985); and filled circles, slender tuna (Sepulveda et al., 2007). The dotted black line represents isothermal conditions (cranial temperature=water temperature).
When the relationship between cranial temperature and myotomal muscle temperature in opah is compared with similar data for other endothermic fishes, the opah is most similar to the shortfin mako shark, Isurus oxyrinchus, and overlaps with some of the tunas, including Allothunnus fallai, which has specialized heater tissue (Fig. 6). In shortfin mako and porbeagle (Lamna nasus) sharks, Tx values ranged from 0.7°C to 6.7°C, with a mean of 2.8°C (Block and Carey, 1985). In tunas, Tx values range from 1.5°C in the black skipjack, Euthynnus lineatus, to 15.5°C in giant Atlantic bluefin, Thunnus thynnus (Stevens and Fry, 1971; Linthicum and Carey, 1972; Schaefer, 1984; Schaefer, 1985; Sepulveda et al., 2007). In all groups, Tx is greater at lower ambient temperatures (Fig. 6) suggesting the capacity to modulate Tx. Unfortunately, static values do not indicate the extent to which cranial temperature may be buffered from ambient temperature changes in vivo and Tx will probably vary substantially throughout a dive, as has been recorded by acoustic telemetry in the swordfish (Carey, 1990).
Based on anatomical, histological and biochemical characteristics, we have provided evidence that the PLRM is the primary source of heat for cranial endothermy in the opah. The PLRM is in the center of the cranial cavity, adjacent to the brain and is the largest extraocular muscle. It is darker red in color and has a higher CS activity, indicating a higher capacity for aerobic heat production, than the distal lateral rectus extraocular muscle, the other extraocular muscles and the locomotor muscles.
We have also identified features that could function to retain the heat produced by contraction of the PLRM. First, the numerous parallel arteries branching from the carotid arteries, each surrounded by veins, should conserve metabolic heat and minimize convection of heat to the gills. The diameters of the heat exchanger blood vessels that we measured in one opah are similar to those reported for the cranial heat exchangers in other species, including the Atlantic bluefin tuna, Thunnus thynnus [80–120 μm for arteries and 40–150 μm for veins (Linthicum and Carey, 1972)], the slender tuna, Allothunnus fallai [50–100 μm (Sepulveda et al., 2007)], the blue marlin, Makaira nigricans [100–300μm for arteries and 80–180μm for veins (Block, 1987a)] and the shortbill spearfish, Tetrapturus angustirostris [100 μm for arteries and 60 μm for veins (Block, 1987a)]. Second, the fat layer overlaying the opisthotic bone reduces conductive heat loss from the PLRM to the gill cavity. Third, the fat surrounding the back of the eyeball provides further insulation. The position of the PLRM within the cranial cavity would allow the heat generated by this muscle to warm the brain by conduction across the thin layer of bone that lies in between them.
In addition to the PLRM, our evidence suggests that the PSRM may also contribute to cranial endothermy. The proximal portion of the SRM is ventral to the braincase and is perfused by a putative counter-current heat exchange system. However, the lower CS activity of the PSRM (Table 2), its smaller size (Table 1) and less developed heat exchanger indicate that the contribution of this muscle to cranial endothermy would be less than that of the PLRM. These same two muscles are modified as heater tissue in billfishes and the butterfly mackerel, respectively, most probably due to their proximity to the brain, optic nerve and eyes.
As the opah swims in the labriform locomotor mode, using the pectoral fins rather than the axial muscle for routine propulsion (Rosenblatt and Johnson, 1976), the head moves up and down with each swimming stroke. Contraction and relaxation of the extraocular muscles most probably compensates for this recoil, and one might expect that this compensation would involve greater contractile activity in the superior and inferior extraocular muscles. However, we propose that it is the lateral rectus that is most important in elevating cranial temperature in the opah because it is the largest of the extraocular muscles with the highest aerobic capacity, is located beneath the brain and is perfused by a large putative heat exchanger.
Our results demonstrate that the opah's extraocular muscles are not modified into specialized heater tissue as has been observed in billfishes, butterfly mackerel and slender tuna. Microscopic observations of the opah PLRM and PSRM indicate that these muscles are composed of normal striated muscle fibers containing myofibrils, as are all other extraocular muscles. The biochemistry supports the same conclusion. The modified heater tissues of billfishes and butterfly mackerel have a much higher CS activity (136–290 units g–1 at 25°C) (Tullis et al., 1991) than the PLRM of opah (17.4–67.6 units g–1 at 20°C). The opah's PLRM CS activity is similar to that in highly aerobic fish muscles that retain their contractile function. For example, the mean CS activity at 20°C in slow-oxidative myotomal muscle of endothermic tunas ranges from 43.4 to 69.8 units g–1 and that of ectothermic scombrids ranges from 23.2 to 51.1 units g–1 (Dickson, 1996; Korsmeyer and Dewar, 2001). The lower CS activity in the distal region of the LRM (Table 2) most probably results from differences in fiber type composition along the length of the muscle (Tullis and Block, 1997). Even though the extraocular muscles are not modified as heater tissue, it is possible that enough heat is produced by contraction of the PLRM and PSRM and retained by the putative counter-current heat exchangers to maintain elevated cranial temperatures in the opah. The same mechanism of retaining metabolic heat produced by skeletal muscle contraction results in regional endothermy in the cranial region of most tunas and in the axial musculature of tunas and lamnid sharks.
A molecular phylogeny of acanthomorph teleost fishes (Chen et al., 2003) shows that the opah (Order Lampridiformes) is distantly related to billfishes, butterfly mackerel and tunas, all of which are found in the Suborder Scombroidei, Order Perciformes. Based on this phylogenetic relationship and the principle of parsimony, cranial endothermy almost certainly evolved independently in the opah. The putative counter-current heat exchanger in the opah is located within the cranial cavity whereas the carotid rete of the Atlantic bluefin tuna is located outside of the cranial cavity, on the posterior margin of the prootic bone (Linthicum and Carey, 1972), suggesting the independent origin of these structures in these two fish groups. The opah is the most basal teleost in which evidence of cranial endothermy has been presented and may represent a first step in the evolution of this trait. The opah has only a putative counter-current heat exchanger and adipose tissue to conserve heat produced by contraction of the LRM and SRM whereas all other fish species exhibiting cranial endothermy either have a specialized heater tissue or also elevate slow-oxidative locomotor muscle temperatures (Dickson and Graham, 2004).
Cranial endothermy in opah would most probably allow for vertical niche expansion. The opah, like almost all fish species with cranial endothermy, is a large pelagic visual predator that moves vertically within the water column, experiencing rapid temperature changes (Carey et al., 1971; Carey et al., 1981; Josse et al., 1988; Dagorn et al., 2000; Schaefer and Fuller, 2002; Fritsches et al., 2003; Musyl et al., 2003; Brill et al., 2005; Fritsches et al., 2005; Polovina et al., 2008). The opah, like other fishes that make deep dives (e.g. swordfish, thresher shark and bigeye tuna), is able to spend protracted periods at depth below the thermocline where it is probably foraging in association with the deep scattering layer (Carey, 1990; Dagorn et al., 2000; Polovina, 2003; Polovina et al., 2008). It has been hypothesized that stable cranial temperatures in other fishes reduce the effects of rapid ambient temperature change on the central nervous system and that elevated retina and brain temperatures enhance the detection of fast-moving prey (Carey, 1982; Block, 1987b; Carey, 1990; Block and Finnerty, 1994; Fritsches et al., 2005; Van den Burg et al., 2005). Recent studies in the swordfish showed that elevated cranial temperature improves temporal resolution by increasing the flicker fusion frequency (Fritsches et al., 2005). Cranial endothermy may result in similar benefits in the opah but future studies are needed to determine if temperature affects the flicker fusion frequency and temporal resolution in this species. Additional studies are also required to determine the extent to which cranial temperatures are elevated and how much cranial temperature changes during dives in free-swimming opah.
LIST OF ABBREVIATIONS
- ANOVA
analysis of variance
- CS
citrate synthase
- CSUF
California State University Fullerton
- DLRM
distal region of the lateral rectus extraocular muscle
- FL
fork length
- IOM
inferior oblique extraocular muscle
- IRM
inferior rectus extraocular muscle
- LRM
lateral rectus extraocular muscle
- MRI
magnetic resonance imaging
- MRM
medial rectus extraocular muscle
- PLRM
proximal region of the lateral rectus extraocular muscle
- PSRM
proximal region of the superior rectus extraocular muscle
- s.d.
standard deviation
- s.e.m.
standard error of the mean
- SOM
superior oblique extraocular muscle
- SRM
superior rectus extraocular muscle
- TEM
transmission electron microscopy
- Tm
myotomal muscle temperature
- Tx
excess temperature=cranial temperature–Tm
We thank the captain and crew of the F/V Sea Pearl and Steve Foltz and Richard Traylor from Chesapeake Fish Company, San Diego, CA, for access to opah. Dan Cartamil, Nick Wegner and Scott Aalbers assisted with collecting chub mackerel. We are indebted to Dr Sean Walker for assistance with software, Steve Karl for microscopy expertise and assistance, Cameron Perry who completed the MRI segmentations, and Dr Robert Koch and Dr Bill Hoese for comments on earlier drafts. This research was supported by the NIH Minority Scientist Development Program at California State University Fullerton (#R25 GM56820), a California State University Fullerton Senior Faculty Research Award to K.A.D., an NSF grant to L.R.F. for the Digital Fish Library (#DBI-0446389), and the University of Hawaii Pelagic Fisheries Research Program under Cooperative Agreement NA17RJ12301 from NOAA. Deposited in PMC for release after 12 months.
References
- Alexander, R. L. (1998). Blood supply to the eyes and brain of lamniform sharks (Lamniformes). Zool. Soc. Lond. 245, 363-369. [Google Scholar]
- Bernal, D. and Sepulveda, C. (2005). Evidence for temperature elevation in the aerobic swimming musculature of the common thresher shark, Alopias vulpinus. Copeia 2005, 146-151. [Google Scholar]
- Block, B. A. (1986). Structure of the brain and eye heater tissue in marlins, sailfish, and spearfishes. J. Morphol. 190, 169-189. [DOI] [PubMed] [Google Scholar]
- Block, B. A. (1987a). Brain and eye warming in billfishes (Istiophoridae): the modification of muscle into a thermogenic tissue. PhD thesis. Duke University, NC, USA, pp. 235.
- Block, B. A. (1987b). Strategies for regulating brain and eye temperatures: a thermogenic tissue in fish. In Comparative Physiology: Life in the Water and on Land (ed. P. Dejours, L. Bolis, C. R. Taylor and E. R. Weibel), pp. 401-420. Padova, Italy: Liviana Press.
- Block, B. A. (1990). Physiology and ecology of brain and eye heaters in billfishes. In Planning the Future of Billfishes (ed. R. H. Stroud), pp. 123-136. Savannah, GA: National Coalition for Marine Conservation.
- Block, B. A. (1991). Endothermy in fish: thermogenesis, ecology and evolution. In Biochemistry and Molecular Biology of Fishes, Vol. 1 (ed. P. W. Hochachka and T. P. Mommsen), pp. 269-311. New York: Elsevier. [Google Scholar]
- Block, B. A. and Carey, F. G. (1985). Warm brain and eye temperatures in sharks. J. Comp. Physiol. B 156, 229-236. [DOI] [PubMed] [Google Scholar]
- Block, B. A. and Finnerty, J. R. (1994). Endothermy in fishes: a phylogenetic analysis of constraints, predispositions, and selection pressures. Environ. Biol. Fishes 40, 283-302. [Google Scholar]
- Block, B. A., Copeland, E. and Carey, F. G. (1982). Fine structure of tissue warming the brain and eye in tuna. Biol. Bull. 163, 356. [Google Scholar]
- Block, B. A., Finnerty, J. R., Stewart, A. F. R. and Kidd, J. (1993). Evolution of endothermy in fish: mapping physiological traits on a molecular phylogeny. Science 260, 210-214. [DOI] [PubMed] [Google Scholar]
- Brill, R. W., Bigelow, K. A., Musyl, M. K., Fritsches, K. A. and Warrant, E. J. (2005). Bigeye tuna (Thunnus obesus) behavior and physiology and their relevance to stock assessment and fishery biology. Collective Volume of Scientific Papers, International Commission for the Conservation of Atlantic Tunas 57, 142-161. [Google Scholar]
- Carey, F. G. (1982). A brain heater in swordfish. Science 216, 1327-1329. [DOI] [PubMed] [Google Scholar]
- Carey, F. G. (1990). Further acoustic telemetry observations of swordfish. In Planning the Future of Billfishes. (ed. R. H. Stroud), pp. 103-122. Savannah, GA: National Coalition for Marine Conservation.
- Carey, F. G. and Lawson, K. D. (1973). Temperature regulation in free-swimming bluefin tuna. Comp. Biochem. Physiol. 44A, 375-392. [DOI] [PubMed] [Google Scholar]
- Carey, F. G., Teal, J. M., Kanwisher, J. W., Lawson, K. D. and Beckett, K. S. (1971). Warm-bodied fish. Am. Zool. 11, 137-145. [Google Scholar]
- Carey, F. G., Teal, J. M. and Kanwisher, J. W. (1981). The visceral temperatures of mackerel sharks (Lamnidae). Physiol. Zool. 54, 334-344. [Google Scholar]
- Carey, F. G., Casey, J. G., Pratt, H. L., Urquhart, D. and McCosker, J. E. (1985). Temperature, heat production and heat exchange in lamnid sharks. Memoir South Calif. Acad. Sci. 9, 92-108. [Google Scholar]
- Chen, W. J., Bonillo, C. and Lecointre, G. (2003). Repeatability of clades as a criterion of reliability: a case study for molecular phylogeny of Acanthomorpha (Teleostei) with larger number of taxa. Mol. Phylogenet. Evol. 26, 262-288. [DOI] [PubMed] [Google Scholar]
- Dagorn, L., Bach, P. and Josse, E. (2000). Movement patterns of large bigeye tuna (Thunnus obesus) in the open ocean, determined using ultrasonic telemetry. Mar. Biol. 136, 361-371. [Google Scholar]
- Dickson, K. A. (1996). Locomotor muscle of high-performance fishes: what do comparisons of tunas with ectothermic sister taxa reveal? Comp. Biochem. Physiol. 113A, 39-49. [Google Scholar]
- Dickson, K. A. and Graham, J. B. (2004). Evolution and consequences of endothermy in fishes. Physiol. Biochem. Zool. 77, 998-1018. [DOI] [PubMed] [Google Scholar]
- Dickson, K. A., Gregorio, M. O., Gruber, S. J., Loefler, K. L., Tran, M. and Terrell, C. (1993). Biochemical indices of aerobic and anaerobic capacity in muscle tissues of California elasmobranch fishes differing in typical activity level. Mar. Biol. 117, 185-193. [Google Scholar]
- Finnerty, J. R. and Block, B. A. (1992). Convergent evolution of regional endothermy in teleosts: dissection of the butterfly mackerel. Am. Zool. 32, 142A. [Google Scholar]
- Fritsches, K. A., Marshall, J. N. and Warrant, E. (2003). Retinal specializations in the blue marlin: eyes designed for sensitivity to low light levels. Mar. Freshw. Res. 54, 333-341. [Google Scholar]
- Fritsches, K. A., Brill, R. W. and Warrant, E. J. (2005). Warm eyes provide superior vision in swordfishes. Curr. Biol. 15, 55-58. [DOI] [PubMed] [Google Scholar]
- Gon, O. (1990). Lampridae. In Fishes of the Southern Ocean (ed. O. Gon and P. C. Heemstra), pp. 215-217. Grahamstown, South Africa: J. L. B. Smith Institute of Ichthyology.
- Josse, E., Bach, P. and Dagorn, L. (1998). Simultaneous observations of tuna movements and their prey by sonic tracking and acoustic surveys. Hydrobiologia 371, 61-69. [Google Scholar]
- Korsmeyer, K. E. and Dewar, H. (2001). Tuna metabolism and energetics. In Tuna, Physiology, Ecology, and Evolution. Fish Physiology, Vol. 19 (ed. B. A. Block and E. D. Stevens), pp. 35-78. San Diego: Academic Press. [Google Scholar]
- Linthicum, D. S. and Carey, F. G. (1972). Regulation of brain and eye temperatures by the bluefin tuna. Comp. Biochem. Physiol. 43A, 425-433. [DOI] [PubMed] [Google Scholar]
- Musyl, M. K., Brill, R. W., Boggs, C. H., Curran, D. S., Kazama, T. K. and Seki, M. P. (2003). Vertical movements of bigeye tuna (Thunnus obesus) associated with islands, buoys, and seamounts near the main Hawaiian Islands from archival tagging data. Fisheries Oceanography 12, 152-169. [Google Scholar]
- Palmer, G. (1986). Lamprididae. In Fishes of the North-eastern Atlantic and the Mediterranean (ed. P. J. P. Whitehead, M. L. Bauchot, J. C. Hureau, J. Nielsen and E. Tortonese), pp. 725-726. Paris: UNESCO.
- Polovina, J. J. (2003). Studying pelagics: discovering the long distance migration and deep diving behavior for large pelagics in the central North Pacific with pop-up archival transmitting tags (part I). http://oceanexplorer.noaa.gov/projects/02census/pelagics/pelagics.html.
- Polovina, J. J., Hawn, D. and Abecassis, M. (2008). Vertical movements and habitat of opah (Lampris guttatus) in the central North Pacific recorded with pop-up archival tags. Mar. Biol. 153, 185-193. [Google Scholar]
- Rosenblatt, R. H. and Johnson, G. D. (1976). Anatomical considerations of pectoral swimming in the opah, Lampris guttatus. Copeia 1976, 367-370. [Google Scholar]
- Schaefer, K. M. (1984). Swimming performance, body temperatures and gastric evacuation times of the black skipjack Euthynnus lineatus. Copeia 1984, 1000-1005. [Google Scholar]
- Schaefer, K. M. (1985). Body temperatures in troll caught frigate tuna, Auxis thazard. Copeia 1985, 231-233. [Google Scholar]
- Schaefer, K. M. and Fuller, D. W. (2002). Movements, behavior, and habitat selection of bigeye tuna (Thunnus obesus) in the eastern equatorial Pacific, ascertained through archival tags. Fishery Bulletin USA 100, 765-788. [Google Scholar]
- Schaefer, K. M., Fuller, D. W. and Block, B. A. (2007). Movements, behavior, and habitat utilization of yellowfin tuna (Thunnus albacares) in the northeastern Pacific Ocean, ascertained through archival tag data. Mar. Biol. 152, 503-525. [Google Scholar]
- Sepulveda, C. A., Dickson, K. A., Frank, L. and Graham, J. B. (2007). Cranial endothermy and a novel brain heater in the most basal tuna species, Allothunnus fallai. J. Fish Biol. 70, 1720-1733. [Google Scholar]
- Stevens, E. D. and Fry, F. E. J. (1971). Brain and muscle temperatures in ocean caught and captive skipjack tuna. Comp. Biochem. Physiol. 38A, 203-211. [Google Scholar]
- Tubbesing, V. A. and Block, B. A. (2000). Orbital rete and red muscle vein anatomy indicate a high degree of endothermy in the brain and eye of the salmon shark. Acta Zool. 81, 49-56. [Google Scholar]
- Tullis, A. and Block, B. A. (1997). Histochemical and immunohistochemical studies on the origin of the blue marlin heater cell phenotype. Tissue Cell 29, 627-642. [DOI] [PubMed] [Google Scholar]
- Tullis, A., Block, B. A. and Sidell, B. D. (1991). Activities of key metabolic enzymes in the heater organs of scombroid fishes. J. Exp. Biol. 161, 383-403. [DOI] [PubMed] [Google Scholar]
- Van den Burg, E. H., Peeter, R. R., Merhoye, M., Meek, J., Flik, G. and Van der Linden, A. (2005). Brain responses to ambient temperature fluctuations in fish: reduction of blood volume and initiation of a whole-body stress response. J. Neurophysiol. 93, 2849-2855. [DOI] [PubMed] [Google Scholar]
- Wolf, N. G., Swift, P. R. and Carey, F. G. (1988). Swimming muscle helps warm the brain of lamnid sharks. J. Comp. Physiol. 157B, 709-715. [Google Scholar]