Summary
Insectivorous echolocating bats face a formidable array of defenses employed by their airborne prey. One such insect defense is the ultrasound-triggered dive, which is a sudden, rapid drop in altitude, sometimes all the way to the ground. Although many previous studies have investigated the dynamics of such dives and their effect on insect survival rate, there has been little work on how bats may adapt to such an insect defense employed in the middle of pursuit. In this study we investigated how big brown bats (Eptesicus fuscus) adjust their pursuit strategy when flying praying mantises (Parasphendale agrionina) execute evasive, ultrasound-triggered dives. Although the mantis dive occasionally forced the bat to completely abort its chase (25% trials), in a number of cases (75% trials) the bat followed the mantis into the dive. In such cases the bat kept its sonar beam locked onto the target and maneuvered to maintain the same time efficient strategy it adopted during level flight pursuit, though it was ultimately defeated by the dive. This study suggests that although the mantis dive can be effective in evading the bat, it does not always deter the bat from continuing pursuit and, given enough altitude, the bat can potentially capture diving prey using the same flight strategy it employs to intercept prey in level flight.
Keywords: bat, echolocation, evasion, insect, predator–prey
INTRODUCTION
Insectivorous echolocating bats must contend with a variety of general and specific defenses employed by insects to evade predation. General strategies employed by insects against bats include limiting overall time in flight (Fullard and Napoleone, 2001; Miller and Surlykke, 2001), avoiding flying at times when bats are also likely to be in the air, flying erratically, and flying at low altitude (Lewis et al., 1993). Some insects also produce sounds that may serve to jam bat sonar or startle bats (Miller, 1991; Møhl and Miller, 1975). The best known specific strategies are dives and erratic loops performed in response to the ultrasonic signals of bats (Roeder and Treat, 1961; Triblehorn and Yager, 2005a; Yager et al., 1990).
It is possible that bats have, in turn, developed counter-measures to insect defenses, though evidence for this has been controversial. Some studies have suggested that bats counter hearing insects by gleaning instead of hawking (Fenton and Fullard, 1979), and by shifting the energy of their echolocation calls out of the insect's hearing range (Neuweiler, 1989). Other proposed strategies include shortening calls, reducing call intensity or going silent altogether, although these changes also occur during bat attacks on non-hearing prey (Miller and Surlykke, 2001). There are studies, however, which call into question the effectiveness of such call adaptations (Russo et al., 2007; Schmidt et al., 2000).
Previous observations of echolocating bats chasing insects in erratic but level flight have shown that bats employ a strategy well suited to the unpredictable maneuvers of their prey. This strategy, in which the bat maintains a constant absolute target direction (CATD), differs from classical pursuit (Rushton et al., 1998) and constant bearing strategies (Fajen and Warren, 2004), and allows the pursuer to minimize the time required to make contact with an erratically moving target (Ghose et al., 2006). It is, however, not known what kind of adjustments bats make to their flight strategy to deal with rapid vertical plane maneuvers such as ultrasound-triggered dives. In this study we investigate the responses bats make to defeat last-second evasion by diving insects.
Mantises are an excellent example of insects that use a specific anti-bat defense. Many mantises posses a single (cyclopean) ear on the midline of the thorax that is most sensitive to ultrasonic sounds between 25 and 45 kHz (for a review, see Yager, 1999). In response to bat vocalizations, mantises perform turns, turns with dives and power dives. Turns are elicited when the ultrasonic stimulus is weak, corresponding to a distant bat, and turns with dives are observed with stronger ultrasonic stimuli. Power dives, in which the mantis directs the thrust from its wings downward to add to gravitational acceleration, are elicited by more intense vocalizations, corresponding to a nearby, perhaps attacking bat (Yager et al., 1990).
In this study we examined vocalization patterns, flight strategies, and sonar beam aim adjustments of big brown bats, Eptesicus fuscus Beauvois, in response to sudden dives initiated by praying mantises [Parasphendale agrionina Mantidae: Miomantinae: Miomantini (Ehrmann and Roy, 2002)] that served as potential prey in a laboratory flight room. This is the first study to look at short term responses of bats to ultrasound-triggered evasive maneuvers by insects. Studying such reactions helps us understand bat insect-capture behavior and may lead to a more complete understanding of the dynamic interactions between echolocating bats and nocturnal insects.
MATERIALS AND METHODS
Flight room
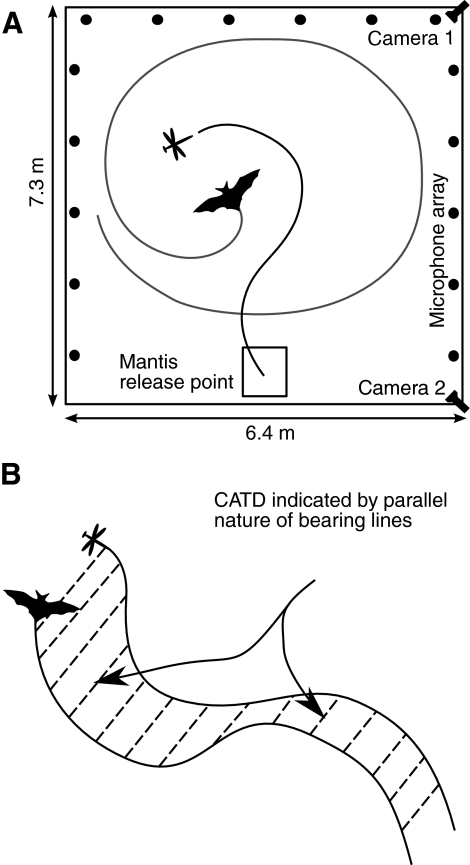
We studied the aerial interaction between big brown bats and praying mantises in a large (7.3 m×6.4 m×2.5 m) laboratory flight room (Fig. 1A). The walls and ceiling of the room were lined with sound absorbent foam to reduce reverberations. The room was illuminated by dim, long wavelength light (>650 nm, normal incandescent bulbs filtered through Plexiglas G #2711; Atofina Chemicals, Philadelphia, PA, USA) to which Eptesicus fuscus (Hope and Bhatnagar, 1979) and at least one species of mantis (Sontag, 1971) are insensitive. Images from two high-speed video cameras (Kodak MotionCorder, CCD-based infrared sensitive cameras, running at 240 frames per second, synchronized to 1/2 frame accuracy) were used to reconstruct the three-dimensional flight paths of the bats and the trajectory of the prey in a calibrated region of the flight room. Two ultrasonic microphones (Ultrasound Advice SM2 microphones and SP2 amplifiers) were placed 30 cm above the floor to record bat vocalizations. A custom-built, U-shaped array of 16 microphones (Knowles FG3329, with custom-built amplifying and filtering circuits) recorded horizontal cross sections of the sonar beam pattern emitted by the bats. This microphone array was used previously to study bat sonar beam directing behavior during target interception (Ghose and Moss, 2003).
Fig. 1.
(A) Schematic of instrumented flight room. The flight room was illuminated with red wavelength lighting. Two high speed infra-red cameras recorded the flights of the bat and mantis. Two ultrasonic microphones placed in the middle of the flight room, 30 cm above the floor, were used to record the vocalizations of the bat. An array of 16 microphones placed round the room was used to record sonar beam patterns emitted by the bats. The mantis was released from the indicated position, from a height of 2 m. (B) Graphical illustration of constant absolute target direction (CATD) pursuit. The bat adopts a CATD strategy if bearing lines drawn to the target appear parallel in the world (absolute) reference frame. Under this condition the bearing angle can change, but the absolute direction of the bearing vector does not. A pursuit trajectory that maintains CATD minimizes (on average) time to intercept of an unpredictably moving target (Ghose et al., 2006).
Animals
Five big brown bats (Eptesicus fuscus), collected locally in Maryland and kept on a reversed day–night schedule, were used in this study. The bats were trained to capture free-flying mantises in the flight room. The bats hunted for food in the flight room on experimental test days and were fed in their cages on other days. On test days, male Parasphendale agrionina, 7–21 days after their molt to adulthood, were released individually in the laboratory flight room. The mantises were raised in our colony, maintained at 25–30°C and 30–50% relative humidity, with a 14 h day length with normal light cycle. All mantises were housed individually as adults and fed flies twice a week.
Experimental protocol
We designed our experiments to evaluate the behavioral responses of big brown bats when their pursuits were interrupted by the mantis' evasive dive maneuver. We examined flight kinematics, vocalization patterns and sonar beam aim direction close to the time of the mantis dive, focusing on how these quantities changed immediately after the mantis dive. Bat responses to mantis dives were compared with a control group of trials in which the mantis' ultrasound-triggered dive was suppressed and the bat chased non-diving but otherwise erratically flying mantises.
We divided the mantises into two groups. Mantises in the first group were untreated, and mantises in the other group were deafened by applying Vaseline© to the ear (Triblehorn et al., 2008). The deafening treatment suppressed ultrasound-triggered escape responses in 19 out of 22 flights. A given trial randomly involved a deafened or hearing mantis and the experimenters were blind to the condition of the mantis prior to release. Bats would chase both hearing and deafened mantises.
Each bat was tested as it chased a single flying mantis in the room. The bat was first released from its temporary cage and allowed to fly in the room for a variable period (10–30 s) while one experimenter (J.D.T.) stood on a step-ladder and kept the mantis concealed in his hand. The mantis was then released into the room from a height of about 2 m, starting the experimental trial. The trial ended when the mantis was captured or when it landed on the floor or walls of the flight room. Only trials in which the mantis attained stable flight were included in the data set. It is probable that the bats learned the release point of the mantis. However, if the bat hovered or circled near the release point, mantis release was delayed until the bat continued to fly around the room. In most cases the mantis was released when the bat was at the far side of the room, requiring the bat to complete at least a half circuit of the room in order to reach the mantis, by which time the mantis had flown to another point in the flight space. The data analysis required three-dimensional reconstruction of both the bat and mantis flight paths. Therefore, only trials in which the encounter occurred in view of both video cameras and within the camera calibrated space of the flight room were used.
Flight path analysis
The flight paths of the bat and mantis were reconstructed using commercially available motion analysis software (Motus, Peak Performance Technologies Centennial, CO, USA, now merged to form Vicon Peak). Data analysis was done using scripts written in MATLAB (MathWorks, Natick, MA, USA). The digitized flight track data points were smoothed using a rectangular sliding window 125 ms long. Flight velocities were obtained using Newton's difference quotient method (Thompson, 1919) to compute time derivatives of position for each time step.
Flight strategy quantification
In previous work it has been shown that bats pursue non-diving insects using a constant absolute target direction (CATD) strategy (Ghose et al., 2006), also known as parallel navigation in the missile guidance literature (Yuan, 1948). In this strategy the bat maneuvers such that bearing lines drawn from the bat to its target appear parallel when viewed from an external reference frame (Fig. 1B). A pursuer and evader pair can maintain parallel navigation (or CATD) both when the distance between them decreases, as well as when it increases. A pursuer will attempt to maneuver so that distance decreases. Conversely, an evader that is aware of its pursuer's position could maneuver to increase distance. In both cases, when the CATD condition is attained, the rate of change in distance between pursuer and evader is the maximum that can be obtained, given the current speed of the pursuer and the evader. The CATD strategy, therefore, enables a faster pursuer to minimize the time it takes to intercept a slower, unpredictably moving target. This is in contrast to other common pursuit strategies reported in the literature such as `classical pursuit' (Rushton et al., 1998) and `constant bearing' (Fajen and Warren, 2004).
We computed an index (γ) of how close the bat's pursuit approached the ideal (CATD) condition (Justh and Krishnaprasad, 2006) and used this to determine the quality of the bat's pursuit. γ is the cosine of the angle between the bat-target separation vector and the vector representing the rate of change of this separation. A γ value of –1 indicates the bat is adhering perfectly to a CATD strategy while closing in at a maximum rate (the separation between them is directly decreasing). By contrast, a γ value of +1 indicates that the target and the bat, while adhering to CATD, are separating at a maximum rate. Intermediate values of γ indicate partial convergence to the CATD strategy. Random motions of two actors that are not reacting to each other would cause random fluctuations of γ between +1 and –1, leading to a time averaged value of γ=0. In our experiments we consider sustained periods of γ=–1 or γ=+1 (combined with specific bat vocal behavior – see next section) as evidence of a systematic interaction between the bat and insect.
Vocalization pattern analysis
Vocalization times were selected by displaying the time-waveform and spectrogram of each call and marking the start and stop times of the fundamental of each bat call. Pulse repetition rate (PRR) was computed from the time interval between the start of successive calls. We used the PRR to identify the beginning and end of periods when the bat was actively pursuing the insect. Typically, when searching or cruising, bats produce calls at low PRR (<20 Hz). Upon detecting and then pursuing prey, bats raise the PRR to 100 Hz, and conclude with rates as high as 150 Hz, referred to as the `buzz'. The vocalization pattern can, therefore, be used to index the behavioral state of the bat (Griffin, 1953; Griffin et al., 1960; Surlykke and Moss, 2000).
Sonar beam direction analysis
Sonar beam directions were computed from sound intensities measured across the array of microphones (Ghose and Moss, 2003). The experimenters were not in control of where the encounter between the bat and mantis took place, and in many of the encounters when video data were available microphone array data were not suitable for sonar beam computation. For this reason, examples of sonar beam directing behavior are presented, but without statistical analysis. In addition, only the horizontal direction of the bat's sonar beam aim is available from the linear microphone array, so we were unable to investigate the vertical tracking behavior of the bat's sonar beam.
Identification of mantis dives
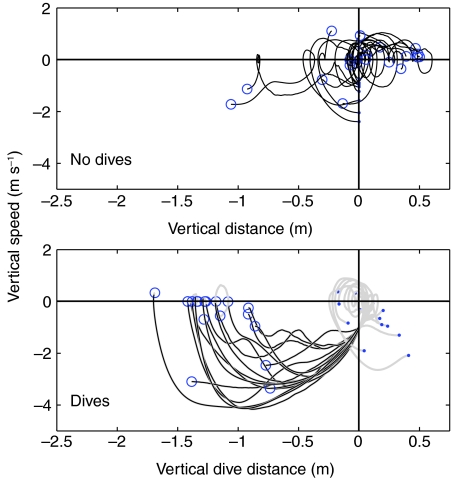
Each mantis trajectory was categorized as `dive' or `no-dive' based on the vertical speed and distance dropped. Fig. 2 shows mantis vertical speed plotted against vertical distance traveled. In this figure negative vertical speed indicates downward motion towards the floor and negative vertical distance indicates a drop in height. A dive was considered to have taken place if a flight segment was found where the mantis' vertical speed always exceeded –1.0 m s–1 and during which the mantis lost more than 0.5 m in height. The mantis dive initiation point was defined as the start of such a segment. Even in level flight, mantises were sometimes observed to make bobbing movements, known as the `goldfinch flight' (Yager et al., 1990). Our criterion was chosen to prevent selection of such bobbing as dives as well as to reject slow descents.
Fig. 2.
Classification of mantis responses into `dive' or `no-dive' based on its flight trajectory. Vertical distance traveled by each mantis is plotted against its corresponding vertical speed. Dive initiation was identified as the start of the first time segment when the downward vertical speed of the mantis continuously exceeded 1 m s–1 and it dropped more than 0.5 m during the same segment. Dive end point is identified as the time when the vertical speed turned positive, or when the mantis hit the ground. Dots show start of trajectory data and circles show end of trajectory data. In the dives plot, sections of the trajectory segmented as a dive are shown in black, whereas the rest of the trajectory is shown in gray.
In Fig. 2, dots and circles indicate the beginning and end of the trajectories, respectively. The top panel shows all the mantis trajectories classified as `no-dives'. Bobbing can be seen as small loops, indicating periodic losses and gains in altitude. The bottom panel shows trajectories classified as dives. The gray part of each trace shows the part of the trajectory prior to dive initiation, and the black part shows post-dive trajectory. Data for most trials were collected up to the point the mantis landed on the floor (Fig. 2; `dive' trajectories that end with vertical speed at or near zero). The mantis dive (shown in black in the lower panel) was often preceded by a slight upward bob (Fig. 2; clockwise loops in the gray traces, just prior to the black segment). This is consistent with earlier observations of mantis dives (Yager et al., 1990).
Caveats related to room height
The height of the flight room was 2.5 m. Bat–mantis interactions in the field have been observed from ground level to tree-top heights (Yager et al., 1990). The comparatively low height of the flight room most probably resulted in the bats not attaining as fast a dive speed as they would in the wild (and in general not flying as fast as they would in the wild). The flight room ceiling height also means our experiments form a sample of interactions within a limited range of elevation above the floor, whereas in the wild such interactions would occur over a much broader range. In this study we focused on the response of the bat immediately after the mantis dive. This part of the bat's response should be minimally affected by the constraints imposed by the dimensions of the room.
RESULTS
In preparation for each flight encounter, a single bat was released into the flight space and allowed to fly circuits for a variable period (10–30 s). While circling, the bat produced sonar pulses at a low rate (20 Hz or less), typically obtained in laboratory experiments when no prey is present in the room. In most cases, shortly after mantis release, the bat would increase its PRR and direct its flight towards the mantis, indicating it had detected and was responding to the flying mantis. We used high PRRs (around 100 Hz) to indicate periods when the bat had committed to insect pursuit (Ghose and Moss, 2006).
The bat–mantis interactions were categorized into four classes, based on the flight behavior of the mantis, the flight responses of the bat, and the vocal patterning produced by the bat. All mantis captures occurred during flights when the mantis did not dive (`captures'). All mantises that executed dives escaped from the bat. After the mantis dived, the bat would sometimes follow the mantis into the dive (`follows'), abort its pursuit (`abort'), or display other behavioral responses (`other'). When pursuing mantises that did not dive, the bats employed a CATD strategy (Ghose et al., 2006). We found that during `aborts' the bat's convergence to a CATD pursuit was broken by the mantis dive. During some `follows' the bat maintained the CATD pursuit for about 400 ms into the dive, before breaking off. During some follows, however, the bat did not manage to converge on a CATD pursuit either before of after the dive.
Four classes of bat-mantis interactions
Captures
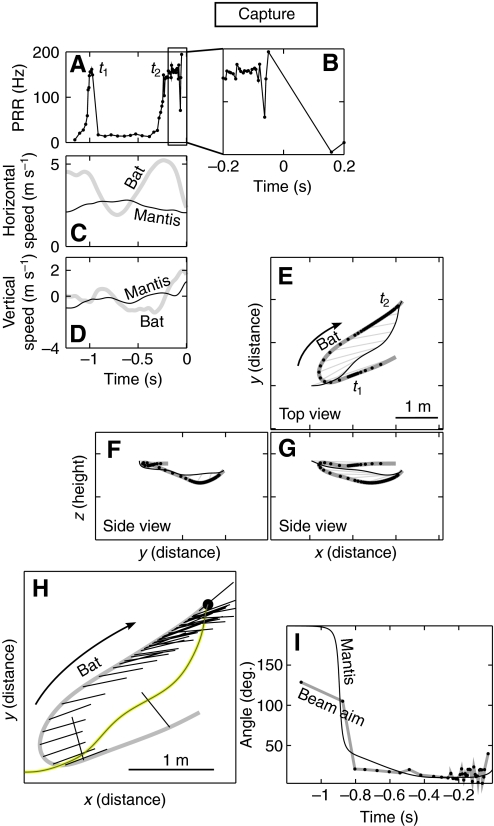
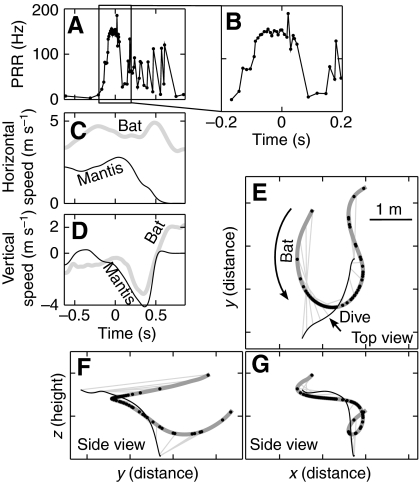
In trials when the mantis did not dive, the bat would execute a typical attack sequence (Griffin, 1958) an example of which is shown in Fig. 3. In this trial the bat initially approached the mantis head-on, at a relative horizontal speed of about 7.5 m s–1 (Fig. 3C; sum of speeds, since bat was approaching the mantis) producing sonar vocalizations at a high PRR, indicative of pursuit. The bat was unable to maneuver fast enough to capture the mantis on the first pass [time (t)=t1] and made a `U-turn' to reposition itself. The U-turn was accompanied by braking (dip in horizontal speed in Fig. 3C; t>t1) and a drop in PRR (Fig. 3A; t>t1). After completing the U-turn, the bat accelerated and resumed its pursuit of the mantis, once again increasing its PRR. The bat locked its sonar beam onto the target during the entire maneuver, starting with the U-turn and continuing up to capture (Fig. 3H, black lines drawn from bat trajectory; and Fig. 3I), consistent with a previous study of bats capturing tethered prey (Ghose and Moss, 2003). The bearing lines (Fig. 3E–G, light gray lines) drawn from bat to target were parallel to each other during the final phase of capture. The parallel nature of the bearing lines shows that during target interception the bat adopted a constant absolute target direction (CATD) strategy (Ghose et al., 2006). Right after capture the bat abruptly dropped its PRR (Fig. 3B; t>0 s). During this encounter, neither the mantis nor the bat made large maneuvers in the vertical plane (Fig. 3D,F,G). The bat's vocalization pattern resembles that reported for aerial hawking sequences in the lab and field (Griffin, 1958; Surlykke and Moss, 2000), as does its sonar beam tracking behavior (Ghose and Moss, 2003). Such interactions were classified as `captures'. In our experiments, whenever the mantis did not dive, the bat was successful in capturing the mantis.
Fig. 3.
Example of a capture. In this trial the mantis was manipulated to suppress the ultrasound-triggered dive. (A,B) Pulse rate (B is an expanded view of the boxed region in A), (C) horizontal speed and (D) vertical speed during the trial. (E) Top view and (F,G) side views of the flight trajectory of a bat (thick gray line) and a mantis (black line). Thin gray lines drawn from bat to mantis are the bearing lines. (H) Top view with sonar beam axis direction (black lines) overlaid and (I) compared with bearing to target. In E–G the bat approached the mantis, over shot it (t1) and then turned back to capture it. Gray bearing lines are drawn from the bat to the mantis every 100 ms, illustrating the parallel navigation strategy adopted by bats as they pursue prey (Ghose et al., 2006). In C during the chase, except for the sharp turn after the overshoot, the bat was faster than the mantis in the horizontal plane. The pulse rate plot in A shows that the bat made a short buzz during the overshoot (t1) and then again during the capture (t2); B shows the abrupt drop in pulse rate after the capture (which occurred at time t=0). H and I show that the bat kept its sonar beam locked onto the target throughout the chase.
Aborts
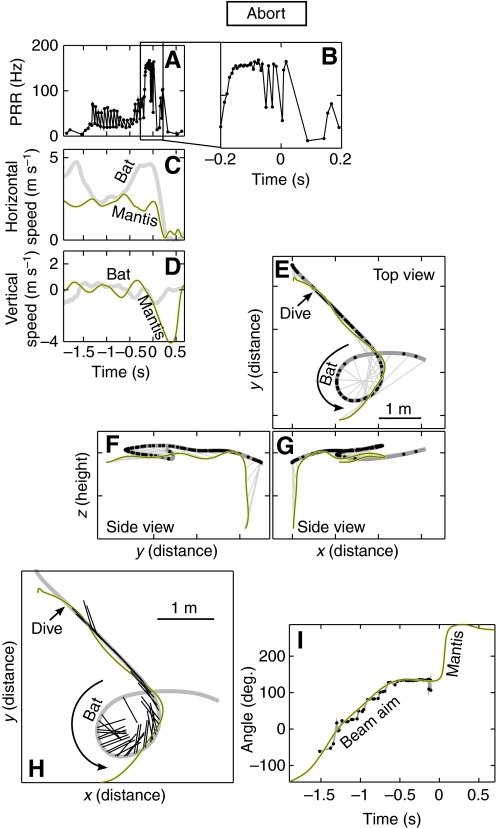
In contrast, for some trials when the mantis dived, the bat would abruptly terminate its pursuit shortly after mantis dive initiation, as illustrated in Fig. 4. Here the bat performed an almost complete horizontal loop to maneuver itself into a `tail-chase' with the mantis. The bat's horizontal speed dropped during the initial loop (Fig. 4C; dip at t=–1.5 s), after which it accelerated and approached the mantis from directly behind. During the loop and right up to the mantis dive the bat kept its sonar beam locked onto its target (Fig. 4H,I). There was little change in the vertical (Fig. 4C) or horizontal (Fig. 4D) speeds of the mantis until t=0 s, at which point the mantis dived. Up to this point the bat had matched or exceeded the mantis' horizontal speed and was about to capture the mantis. The dive, indicated by a sudden increase in downward vertical speed in Fig. 4C (t=0 s), caused the mantis to drop away rapidly from the bat just before capture. In response to this maneuver the bat dropped its PRR and continued on a level course without attempting to compensate for the mantis dive. The bat's vocal, flight and sonar beam behavior was typical for insect captures right up to the mantis dive point, after which the bat stopped responding to the mantis. Such interactions were classified as `aborts'.
Fig. 4.
Example of an abort. (A,B) Pulse rate (B is an expanded view of the boxed region in A), (C) horizontal speed and (D) vertical speed during the trial. (E) Top view and (F,G) side views of the flight trajectory of a bat (thick gray line) and a mantis (black line). The dive point is indicated by a black arrow. The bat entered a buzz but aborted it shortly after the mantis dived (t=0). (H,I) The bat kept the sonar beam locked on target right up to the abort point.
Follows
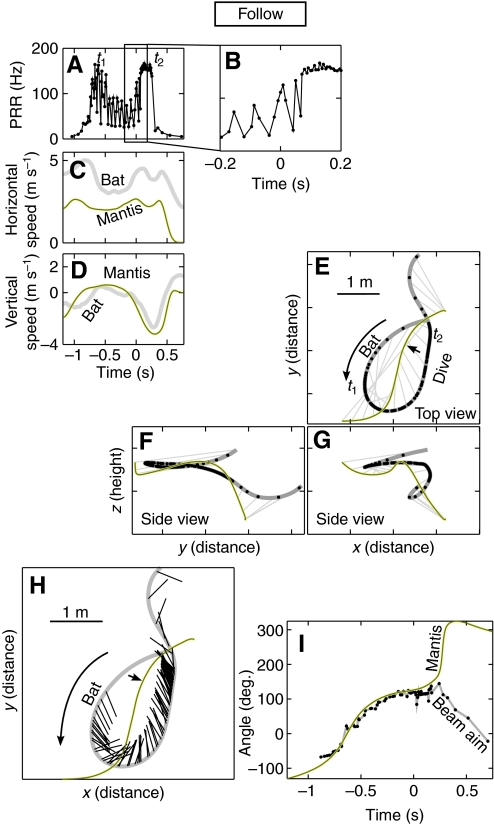
In other trials when the mantis dived, the bat would attempt to compensate for the mantis dive and persist in its pursuit until the mantis got close to the floor, at which time the bat broke off its attack. An example of such a trial is illustrated in Fig. 5. Here the bat approached the mantis producing a high PRR (Fig. 5A), performed a U-turn that reduced its horizontal speed (Fig. 5C; dip in bat speed, t=t1) and then accelerated again to catch up with the mantis (Fig. 5C; bump in bat speed t=t2). At time t=0, the mantis dived (Fig. 5D; increase in mantis vertical speed downward, t=0) and the bat, instead of breaking off its attack as in the previous example, also adjusted its vertical speed and dived after the mantis (Fig. 5D; increase in bat vertical speed downward which follows and almost matches mantis dive). The bat continued to pursue the mantis 200 ms into the dive before ending its chase and pulling up about 1 m from the floor. During the entire chase, including part of the mantis dive, the bat locked the horizontal direction of its sonar beam on the target (Fig. 5H,I) and maintained a CATD strategy (Fig. 5E; parallel nature of bearing lines). The bat's vocal, flight and sonar beam behavior was typical for insect captures up to and beyond the mantis dive point. Typically, in such trials, the bat terminated its chase after the mantis hit the floor. Such interactions were classified as `follows'.
Fig. 5.
Example of a follow. (A,B) Pulse rate (B is an expanded view of the boxed region in A), (C) horizontal speed and (D) vertical speed during the trial. (E) Top view and (F,G) side views of the flight trajectory of a bat (thick gray line) and a mantis (black line). The dive point is indicated by a black arrow. (H,I) The bat kept its sonar beam locked on target about 200 ms into the dive before breaking off. The bat increased its repetition rate (t=–0.7, s=t1) and made a U-turn to chase the mantis. The mantis dived at time t=0, with an abrupt negative swing of the vertical speed (D). The bat continued its high repetition rate phase (t2) and pursued the mantis down, breaking off the pursuit as it approached the floor (F–G).
Other behavior patterns
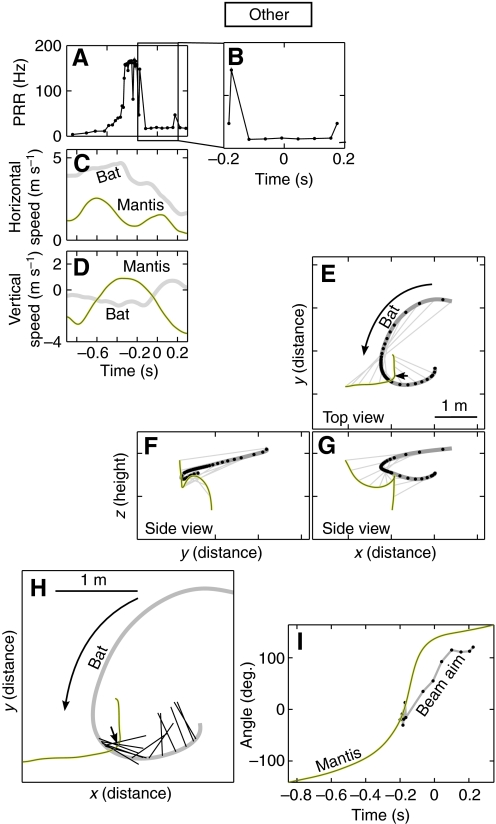
We also observed mantis dives when the bat was not producing high PRRs (indicative of pursuit) during dive initiation. Such interactions consisted of `pre-emptive' dives and `delayed' dives. During pre-emptive dives, the mantis dived for safety before the bat showed behavioral patterns typical of pursuit. During delayed dives, the mantis began its dive after an initial, unsuccessful, attack by the bat, and before the bat returned for a second pass. An example of a `delayed' dive is shown in Fig. 6. Here the bat initially approached the mantis and increased its PRR (Fig. 6A; increased PRR starting at t=–0.3 s). Although sonar beam data is not available for much of the approach, Fig. 6H,I shows that, during the high PRR phase, the bat had locked its sonar beam on the target (five vocalizations, around t=–0.2 s). Shortly after this, however, the bat flew past the mantis, dropped its PRR and directed its sonar beam elsewhere, indicating it had broken off the attack. The mantis dived 200 ms later. In all these trials the mantis dive did not occur in the middle of a bat attack, and did not allow us to investigate the local effects of the dive on bat pursuit. These trials were classified as `other'.
Fig. 6.
Example of a trial classified as `other'. (A,B) Pulse rate (B is an expanded view of the boxed region in A), (C) horizontal speed and (D) vertical speed during the trial. (E) Top view and (F,G) side views of the flight trajectory of a bat (thick gray line) and a mantis (black line). The dive point is indicated by a black arrow. (H,I) The bat probed the mantis with a sonar beam during high repetition rate phase (t<=–0.2 s) but terminated this phase and directed its sonar beam away both before and after the dive.
Summary of data in the four classes
We analyzed a total of 35 trials of which 18 (51%) were no-dive and 17 (49%) were dive trials. In the dive category we made further subdivisions based on the bat's behavior – abort, follows and other – as described in the examples above. In two trials (2/17, 12%) the bat aborted its pursuit, by abruptly terminating its high PRR, within 50 ms of the mantis dive (`abort'). In six trials (6/17, 35%) the bat continued producing high PRRs at least 200 ms into the mantis dive, indicating continued pursuit even after the dive (`follow'). In nine trials (9/17, 53%) the mantis dive occurred at a time when the bat was not producing high PRRs (`other'). A detailed count of behavioral responses across bats is provided in Table 1.
Table 1.
Distribution of bat–mantis encounters grouped by individual bat
|
Dive (mantis evaded capture)
|
|||||
|---|---|---|---|---|---|
| Bat ID | Abort | Follow | Other | No dive (mantis captured) | Total trials |
| A | 0 | 2 | 3 | 5 | 10 |
| B | 1 | 0 | 1 | 2 | 4 |
| C | 0 | 0 | 2 | 0 | 2 |
| D | 1 | 3 | 2 | 6 | 12 |
| E | 0 | 1 | 1 | 5 | 7 |
The total number of trials run with each bat is the sum of the `dive' and `no-dive' columns. All mantis captures occurred in trials in which the mantis did not dive. Of the trials in which the mantis dived to escape, the bat aborted the chase (abort), followed the mantis into the dive (follow) or displayed other responses, including not committing to the chase (other)
Evasive behavior of the mantis
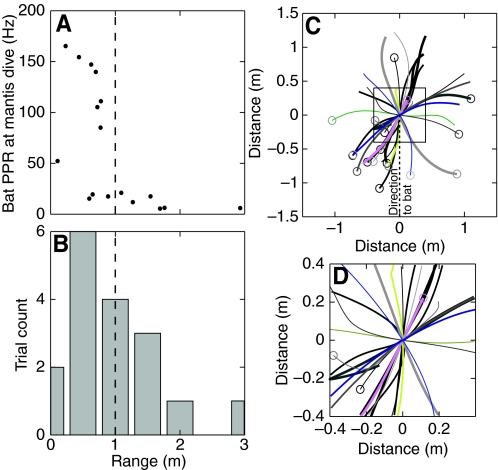
Mantis dive initiation was triggered by a wide variety of bat vocalization patterns and occurred at a wide range of distances from the bat. Fig. 7A shows the pulse rate of the bat just prior to mantis dive, plotted against bat–mantis range at dive initiation, and Fig. 8A (trials in the `dives' section) shows the pulse pattern produced by the bat plotted with respect to the mantis dive time (t=0). In some trials the bat never vocalized at a rate above 20 Hz in a 1 s window preceding the dive (seventh trial from top under `other') while in other trials the bat persistently pursued the insect, producing high PRR calls for almost 1 s prior to the dive (second trial from top under `follows'). Fig. 7B is a histogram of the distance between the bat and the prey at the time when the mantis dive occurred. Mantis dives were initiated more frequently at closer ranges, with 70% (12/17) of dives occurring within 1.0 m from the bat.
Fig. 7.
Parameters of mantis dive. (A) Pulse repetition rate (PRR) of the bat at the time of the mantis dive, plotted against range of mantis from the bat at dive time. Mantis dives occurred during both low and high PRRs. (B) Distribution of mantis dive ranges follows a Poisson like pattern, with 71% of dives (12/17) occurring within 1.0 m (dashed line) from the bat. (C) Top view of mantis flight tracks, rotated and translated so that the dive point is at (0,0) and the direction of flight is relative to the position of the bat at the dive point. In this plot, a trajectory pointing up (along the y-axis) at the dive point indicates the mantis was flying directly away from the bat at the time of the dive. (D) Enlargement of boxed region in C illustrating that in all but two dives the mantis did not make a sharp turn during the dive, and that the mantis dive was not directionally dependent on the position of the bat.
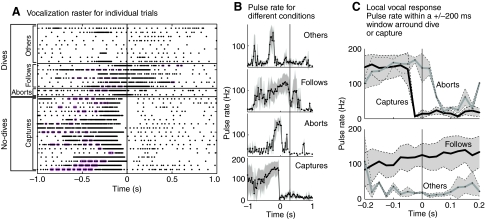
Fig. 8.
Plot of vocalization patterns for 35 trials. (A) Vocalization rasters. Time t=0 is either capture point, or mantis dive point. Pink shading demarcates strobe groups. (B,C) Mean (line) and standard deviation (shaded area) of PRR for the four categories of trials. In 18 of these trials the mantis did not dive and was caught by the bat (`no-dives' or `captures'). In the other 17 trials the mantis dived in response to the bat vocalizations (`dives') and evaded capture. `Aborts' are trials where the bat cut off its buzz abruptly just after the dive. `Follows' are trials where the bat continued its high repetition rate phase even after the mantis dive. `Others' are trials where the mantis dived while the bat was not producing pulses at a high repetition rate.
Consistent with previous experiments using artificial bat sound generators to elicit evasive behaviors by mantises (Yager et al., 1990), we observed that mantis dives were not directional relative to the position of the bat near dive time (Fig. 7C,D). Fig. 7C shows top-views of the 17 mantis trials in which we observed a dive. Each line shows mantis flight trajectory data up to 400 ms before and after the dive. Each trajectory is a different colour and line thickness, and the start position is indicated by a circle. The trajectories themselves have been translated to place the dive point at the center of the axes (0,0) and rotated so that the position of the bat relative to the mantis heading at dive time is along the black dotted line directed along co-ordinates 0,–1. Fig. 7D is a larger scale view of the boxed region in Fig. 7C. In such a representation, if the mantis dive was directionally dependent on the bat position, then each trajectory, around the time it crossed the origin of the axis (near dive point), would deflect in a systematic way with reference to the black dotted line (direction to the bat). Fig. 7C,D shows, however, that in almost all cases the mantis continued flying in the same horizontal direction before and after the dive. Post-dive turning rate traces rose 3 standard deviations above the mean pre-dive turning rate for only two trials (equivalent to a P value of 0.001).
Vocalization behavior of the bat
The vocalization behavior of the bats across 35 trials is summarized in Fig. 8. Trials are classified as `captures', `follows', `aborts' and `other', based on the mantis and bat behavior as described above. Vocalization rasters for the trials are shown in Fig. 8A, grouped by category. In this panel each row represents a single trial, with time on the x-axis relative to capture or the mantis dive initiation. Each dot represents a vocalization emitted by the bat and a high density of dots indicates a high PRR. The mean and standard deviation of the PRR plotted against time for all trials in a particular behavioral category is shown in Fig. 8B. Fig. 8C shows an expanded view of the PRR profile ±200 ms around dive (or capture) time. Captures, follows and aborts are characterized by having a high PRR just prior to dive/capture time. During captures, the PRR dropped just prior to capture-point, for aborts the PRR dropped just after dive initiation, whereas for follows, a high value for PRR persisted even 200 ms into the dive. `Others' are characterized by a low PRR (typical of the post-capture, post-abort periods) over a ±200 ms window around the dive. There was, however, a bump in PRR ending just 200 ms prior to the dive point. This indicates that, typically, the bat probed the mantis at some point earlier in the trial (exceptions can be seen in Fig. 8A), but was not pursuing it just before dive initiation.
From Fig. 4A and Fig. 5A we note a saw-tooth pattern in the PRR profile before the bat produced the terminal buzz, characterized by a stable PRR of about 150 Hz. This saw-tooth pattern, indicating clusters of pulses produced at a high rate separated by silent periods, has been referred to in previous work as `sonar strobe groups' (Moss et al., 2006; Moss and Surlykke, 2001). We observed strobe groups in all four classes of bat–mantis interactions (Fig. 8A, islands of dots separated by larger gaps).
Bat flight strategy during pursuit
Bats have been shown to pursue both tethered and free flying insects using a CATD flight strategy (Ghose et al., 2006). Under conditions when an evader is moving unpredictably, the pursuer can minimize, on average, the time it takes to catch the evader by adopting a CATD strategy. In this sense, the CATD strategy is time optimal for chasing unpredictably moving prey, and may explain why bats adopt such a pursuit strategy.
A CATD strategy can be informally inferred by inspecting plots of the flight trajectories of the bat and insect and noting whether bearing lines drawn from the bat to the insect are parallel to each other. Such a strategy is evident in the bat flight trajectory plots in Figs 3 and 5. In Fig. 4, we cannot determine whether the bat used a CATD strategy since the bearing lines overlap with each other since the pursuit was a tail-chase.
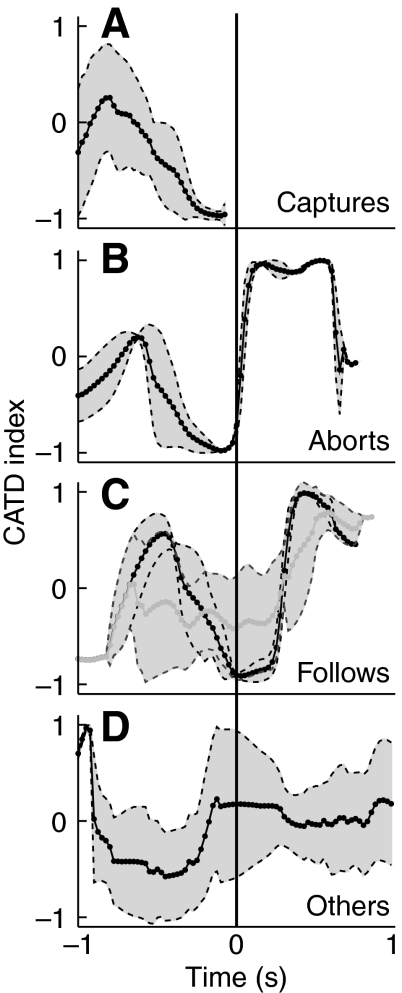
We can compute an index γ to quantify how close the bat is to the ideal CATD strategy (see Materials and methods). A γ value of –1 indicates that the bat is maneuvering to maintain a perfect CATD strategy. Conversely, a γ value of +1 indicates that the separation between the mantis and the bat is increasing at a maximal rate. Fig. 9 shows summaries of γ with time for the different categories of trials. The black line shows the mean value of γ with standard deviation depicted as the surrounding gray band.
Fig. 9.
Pursuit index (γ) for bat–mantis interactions. Gamma is a measure of how close the bat was to a time-optimal pursuit trajectory. γ=–1 indicates a perfectly optimal trajectory, whereas γ=+1 indicates a fully non-optimal trajectory, where the insect was pulling away from the bat (see text for a detailed explanation of γ). (A) γ for captures. The bat maneuvered so that γ → –1 until capture. Limitations of the video data digitization introduce an artifact in the γ computation when the bat gets very close to the target (i.e. just before capture). This artifact has been removed from A, resulting in a gap in the trace just before capture. (B) During aborts γ deviated rapidly from –1 as a result of the dive. (C) During some follows (black line, N=3) the bat kept γ near optimal, before pulling out of the chase. During other follows (gray line, N=3) the bat did not succeed in keeping an optimal γ. (D) During `others' γ was not brought near optimal and the dive did not change the γ trace. Capture/dive time t=0. Mean and standard deviations shown as solid lines and shaded regions, respectively.
During capture trials (Fig. 9A) the bat maneuvered to bring γ close to –1 and maintained this value for 200 ms or more until it merged with the mantis at time t=0. During aborts the bat similarly maneuvered to bring γ=–1 (Fig. 9B). Up to the dive point the profile of γ was similar to that observed during captures. After the dive the value of γ shot up to +1, indicating that the mantis dive was a very effective evasive maneuver. Such trials serve to illustrate the effect of the mantis dive in the absence of any compensatory flight maneuvers by the bat.
In some follows (Fig. 9C, gray line; N=3), even though the high attack PRR was maintained, the bat did not succeed in adjusting γ to –1 before or after the dive. An example of this is shown in Fig. 10 where the bat pursued the mantis into the dive, maintaining a high PRR past the dive point (Fig. 10A,B) and adjusting its vertical speed to match that of the mantis (Fig. 10D; vertical speed traces around t=0). However, as can be seen from the flight trajectory plots, the bat did not converge on a CATD strategy (Fig. 10E; bearing lines are never parallel) either before or after the mantis dive (indicated by an arrow).
Fig. 10.
Example of a poor quality follow. (A,B) Pulse rate (C) horizontal speed and (D) vertical speed during the trial. (E) Top view and (F,G) side views of the flight trajectory of a bat (thick gray line) and a mantis (black line). The dive point is indicated by a black arrow. The bat never managed to converge on a CATD strategy as was typical for captures and other follows. It is probable that the bat did not have enough time to converge on a CATD strategy before the mantis dived.
During trials classified as `other', when the bat's PRR was at a baseline (non-pursuit) level during the dive, the bat did not maneuver to maintain an optimal pursuit (Fig. 9D; γ hovered around zero within a ±100 ms window around the dive). The dip in γ at time t<–200 ms is consistent with the brief increase in PRR around this time (Fig. 8B) indicating that, in some trials, the bat responded to the insect at some point earlier in the trial, but did not pursue it proximate to the dive.
Although the data described so far seem to indicate the bat's flying skills are no match for the diving mantis, the situation is not quite so bleak for the bat. In a subset of follows (Fig. 9C, black line; N=3) the bat not only maneuvered to bring γ=–1 before the dive, it maintained this optimal pursuit even when the mantis dived (plot of γ stayed near –1 even after the dive at t=0). Eventually, about 200 ms after the dive, the bat terminated its pursuit, possibly because the mantis landed on the floor and had disappeared from sonar, or to avoid hitting the floor at a dangerous speed. Though we did not record any captures of hearing mantises resulting from follows, such trials illustrate that the bat can maneuver to counter a mantis dive and the maneuver maintains the advantageous time-optimal CATD strategy that the bat adopts when pursuing targets in level flight.
DISCUSSION
Echolocating bats are challenged by prey that employ a variety of measures to avoid capture. In this study, we focused on how bats adapt their behavior to a prey's sudden evasive maneuver. A previous study demonstrated that big brown bats use a constant absolute target direction (CATD) strategy to chase erratically moving prey in level flight. The CATD strategy is a time-optimal pursuit strategy for unpredictably moving prey. In this study, we found that the mantis' ultrasound-triggered dive rarely forced the bat to abort its chase. In most cases, the bat continued its pursuit, sometimes compensating for the mantis dive and maneuvering to maintain its time-optimal CATD strategy even when the mantis dived. In no case, however, did we observe a bat to successfully capture a diving mantis in these experiments.
The bat does not adjust its flight path in anticipation of the mantis dive
Echolocating bats have been shown to learn specific motion properties of targets and adapt their flight patterns accordingly (Masters, 1988). If the mantis dive occurred in a stereotyped manner, it is possible that the bat could learn to anticipate the dive path. For example, a bat could learn to approach from slightly below the mantis and get a head start on the mantis during a dive. The data we collected show no evidence of bats adopting such an anticipatory strategy. We observed great variability in bat–mantis separation (Fig. 7A) and in bat vocalization patterns (Fig. 8A) that preceded a mantis dive, which is consistent with earlier studies showing dive initiation time varies with the rate and intensity of ultrasonic pulses delivered to the mantis (Triblehorn et al., 2008; Triblehorn and Yager, 2005b; Yager et al., 1990). The high variability in mantis dive initiation point makes it difficult for the bat to prepare itself for the mantis dive ahead of time.
During `aborts' the mantis dive causes the bat to suddenly diverge from a CATD pursuit
Abort trials give us insight into the effects the mantis dive has in the absence of any counter-measures by the bat. As expected, after a dive the vertical distance between the bat and the mantis suddenly increased (Fig. 4F,G). Interestingly, the value of γ, carefully brought near –1 by the bat, shot up to +1 (from Fig. 9B). This indicates that the bat initially maneuvered itself into a time-efficient CATD pursuit (γ close to –1), but the mantis dive (resulting in a γ value close to +1) completely turned the tables on the bat, and resulted in a `perfect get-away' for the mantis, mirroring the bat's own time-optimal approach to an erratically moving insect (Ghose et al., 2006).
The bat needs to sense the bearing and possibly the range to its target to maintain a CATD flight path during its approach. The bat is most certainly using its echolocation to determine the position of the mantis during the chase and make adjustments to its flight path with that information. Such continual adjustments based on sensory feedback enable the bat to maintain its CATD strategy (γ close to –1) in the face of erratically flying prey.
The mantis, however, is unlikely to be able to locate the bat using either vision or audition. Spectral sensitivity measures of another mantis species (Tenodera sinsesis) suggest that the prey in this study would not be able to rely on vision under the long wavelength lighting conditions in our flight room to track the bat position (Sontag, 1971), and its cyclopean ear is non-directional (Yager, 1999) (Fig. 7C). Despite not having access to the bat's position, the mantis still generates an effective maneuver to escape in a time-optimal fashion. The effectiveness of the response probably relies on the fact that, prior to the dive, both the bat and the mantis were maneuvering mainly in the horizontal plane. During this phase the velocity vectors of the bat and mantis were directed largely parallel to the horizontal. At the start of the dive (a sudden out of plane maneuver) the velocity vector of the mantis is directed downwards, while the bat's velocity vector is still horizontal. This sudden large angle between the two vectors results in a large value of γ, indicating that, for a given mantis speed, this vertical maneuver results in the fastest increase in distance between bat and mantis, at least, until the bat reacts to add a vertical component to its motion.
Separation between bat and mantis at dive
In these experiments the bats only followed the mantis when it initiated a dive within a range of 0.6 m. Interestingly, mantis dives occurred at a mean range of 0.9 m (with a skew towards shorter ranges). It is possible that, for dives initiated at further or closer ranges, the mantis runs a higher risk of being captured. If the mantis dives when the bat is further away and does not dive all the way to the ground, the bat could have time to adjust its approach to a lower altitude and attack the mantis after the dive. If, however, the mantis dives when the bat is close, it would risk being captured by the bat more often, either because the bat catches it before dive initiation, or because it has not built up sufficient dive speed.
We note that the encounters we studied took place in a confined space where the bat probably has made various adjustments such as slowing flight speed and lowering vocalization intensity. Such adjustments may affect mantis dive initiation, though in different ways. Lower intensity vocalizations might move the dive initiation point closer to the bat, while a slower flight speed means the bat will take longer to close the distance to a mantis about to dive.
Vocalization behavior of the bat and its pursuit behavior
The PRR profiles for `captures' and `aborts' (Fig. 8) look qualitatively similar to typical insect capture profiles recorded from bats pursuing insects under field and laboratory conditions (Surlykke and Moss, 2000). The high PRR phase in `aborts' has a shorter duration and sharper transition time than the other two categories. From the viewpoint of the bat, the mantis dived earlier in the chase during `abort' trials compared with `capture' trials based on the observation that the bat spent less time in the high PRR phase during `abort' trials. Sonar strobe groups were observed in all classes of bat–mantis interactions. The functional role of such strobe groups for the bat is unknown but may facilitate target localization and tracking (Moss et al., 2006). The bat's production of such vocalization patterns may, however, benefit the mantis in bat evasion (Triblehorn et al., 2008; Triblehorn and Yager, 2005b).
The persistent bat
In 75% (6/8) of the dives that occurred when the bat was pursuing the mantis, the bat maintained a high PRR after the mantis dived, indicating it followed the mantis into the dive. All follows occurred when the mantis was within 0.6 m of the bat at dive initiation and the bat was already attacking it. The bats were never observed to commence an attack on an already diving mantis.
In three of the pursuits where the bat followed the mantis into the dive (3/6, 50%) the bat did not succeed in converging to the time-optimal CATD strategy during the attempted capture (γ hovered around 0; Fig. 9C, gray line). In such cases, although the mantis dive did not lead to a sudden spike in γ to +1 (as happened in aborts) the bat did not manage to maneuver to reduce γ to –1 during the dive. In such trials, the bat responded to the mantis dive (e.g. Fig. 10), but did not achieve CATD pursuit. One possibility is that such incomplete follows were due to the mantis executing a dive before the bat had a chance to converge on a CATD pursuit (mantis dived early into pursuit). All trials tended to start out with a high value of γ (Fig. 9) and the bat had to maneuver to reduce γ to –1. If the mantis executed a dive when the bat was still early in the pursuit the bat did not have enough time to converge on a CATD strategy (γ remained high).
In the other three trials (3/6, 50% of follows; Fig. 9C, black line) the bat pursued the mantis with a CATD strategy both before and after the dive. In such trials the bat maneuvered into a favorable situation before the dive. This is indicated by the similarity between the γ curves in Fig. 9A,C (and B) up to t=0, where γ was close to –1. At the point of the dive (t=0) rather than shooting up (as γ did for aborts; Fig. 9B) γ remained close to –1, indicating that the bat had adjusted its flight well to compensate for the sudden vertical maneuver by the mantis. An example of this is seen in Fig. 5. This example illustrates how the bat maneuvered into position, adopted a CATD strategy (observable from the parallel nature of the bearing lines) and maintained it even after the mantis dived (t=0, black arrow). Presumably, the bat pulled out of the dive at the end in order to avoid hitting the floor and injuring itself, or because the mantis had become acoustically `invisible' at the moment it landed on the floor. We are unable to say definitively how beneficial the bat's ability to follow the mantis dive is, since all our encounters were staged in a flight room only 2.5 m high, and the bats aborted their chases as the mantis got close to or hit the floor. In the field, however, bats and mantises can fly anywhere from ground level to treetop height (Yager et al., 1990) and depending on the height of the initial encounter, the bat may have enough time and space to overtake a diving mantis and capture it.
These experiments suggest that mantis dives are not always sufficient to deter the bat in its pursuit. Previous work with deafened mantises has shown that bats adopt a time-optimal strategy to capture non-diving but otherwise erratically moving prey. The experiments reported here show that in several encounters the bat maneuvered and maintained such a time-optimal pursuit strategy even when chasing the mantis into a dive. This time-optimal strategy is likely to increase the bat's chances of catching up with and capturing diving prey in the field, perhaps providing the bat with an effective counter-measure to the insect's ultrasound-triggered dive.
We thank Drs Timothy Horiuchi and P. S. Krishnaprasad for their earlier contributions to the CATD model (see Ghose et al., 2006), which laid the foundation for flight trajectory analyses in this paper. We also thank the three anonymous reviewers for their comments and suggestions. In addition, we wish to express our appreciation to Melinda Byrns and Amaya Perez, who assisted with the flight experiments, Ann Planeta, who helped with video data analysis, and Sachin Vaidya for his help raising the mantises. This work was supported by a UMD-CP Psychology Dept. Jack Bartlett fellowship (K.G.), NIMH (National Research Service Award) Grant F31 MH12025 (J.D.T.), NSF grant IBN-9808859 (D.D.Y.), NSF grant IBN-0111973 (C.F.M.), and NIH grant R01 MH56366 (C.F.M.). Deposited in PMC for release after 12 months.
References
- Ehrmann, R. and Roy, R. (2002). Systematische aufstellung der gattungen. In Mantodea: Gottesanbiterinnen der Welt (ed. R. Ehrmann), pp. 374-378. Munich: Natur und Teil-Verlag.
- Fajen, B. R. and Warren, W. H. (2004). Visual guidance of intercepting a moving target on foot. Perception 33, 689-715. [DOI] [PubMed] [Google Scholar]
- Fenton, M. B. and Fullard, J. H. (1979). Influence of moth hearing on bat echolocation strategies. J. Comp. Physiol. 132, 77-86. [Google Scholar]
- Fullard, J. H. and Napoleone, N. (2001). Diel flight periodicity and the evolution of auditory defences in the Macrolepidoptera Anim. Behav. 62, 349-368. [Google Scholar]
- Ghose, K. and Moss, C. F. (2003). The sonar beam pattern of a flying bat as it tracks tethered insects. J. Acoust. Soc. Am. 114, 1120-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose, K. and Moss, C. F. (2006). Steering by hearing: a bat's acoustic gaze is linked to its flight motor output by a delayed, adaptive linear law. J. Neurosci. 26, 1704-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose, K., Horiuchi, T. K., Krishnaprasad, P. S. and Moss, C. F. (2006). Echolocating bats use a nearly time-optimal strategy to intercept prey. PLoS Biol. 4, 865-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, D. R. (1953). Bat sounds under natural conditions, with evidence for echolocation of insect prey. J. Exp. Zool. 123, 435-466. [Google Scholar]
- Griffin, D. R. (1958). Listening in the Dark: The Acoustic Orientation of Bats and Men. New Haven, CT: Yale University Press.
- Griffin, D. R., Webster, F. A. and Michael, C. R. (1960). The echolocation of flying insects by bats. Anim. Behav. 8, 141-154. [Google Scholar]
- Hope, G. M. and Bhatnagar, K. P. (1979). Electrical response of bat retina to spectral stimulation: comparison of four microchiropteran species. Experientia 35, 1189-1191. [DOI] [PubMed] [Google Scholar]
- Justh, E. W. and Krishnaprasad, P. S. (2006). Steering laws for motion camouflage. Proc. R. Soc. A 462, 3629-3643. [Google Scholar]
- Lewis, F. P., Fullard, J. H. and Morrill, S. B. (1993). Auditory influences on the flight behavior of moths in a nearctic site. 2. Flight times, heights, and erraticism. Can. J. Zool. 71, 1562-1568. [Google Scholar]
- Masters, W. M. (1988). Prey interception: predictive and nonpredictive strategies. In Animal Sonar: Processes and Performance (ed. P. E. Nachtigall and P. W. Moore), pp. 467-470. New York: Plenum Press.
- Miller, L. A. (1991). Arctiid moth clicks can degrade the accuracy of range difference discrimination in echolocating big brown bats, Eptesicus fuscus. J. Comp. Physiol. A. 168, 571-579. [DOI] [PubMed] [Google Scholar]
- Miller, L. A. and Surlykke, A. (2001). How some insects detect and avoid being eaten by bats: tactics and countertactics of prey and predator. Bioscience 51, 570-581. [Google Scholar]
- Møhl, B. and Miller, L. A. (1975). Ultrasonic clicks produced by the peacock butterfly: a possible bat-repellent mechanism. J. Exp. Biol. 64, 639-644. [Google Scholar]
- Moss, C. F. and Surlykke, A. (2001). Auditory scene analysis by echolocation in bats. J. Acoust. Soc. Am. 110, 2207-2226. [DOI] [PubMed] [Google Scholar]
- Moss, C. F., Bohn, K., Gilkenson, H. and Surlykke, A. (2006). Active listening for spatial orientation in a complex auditory scene. PLoS Biol. 4, e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuweiler, G. (1989). Foraging ecology and audition in echolocating bats. Trends Ecol. Evol. 4, 160-166. [DOI] [PubMed] [Google Scholar]
- Roeder, K. D. and Treat, A. E. (1961). Detection and evasion of bats by moths. Am. Sci. 49, 135-148. [Google Scholar]
- Rushton, S. K., Harris, J. M., Lloyd, M. R. and Wann, J. P. (1998). Guidance of locomotion on foot uses perceived target location rather than optic flow. Curr. Biol. 8, 1191-1194. [DOI] [PubMed] [Google Scholar]
- Russo, D., Jones, G. and Arlettaz, R. (2007). Echolocation and passive listening by foraging mouse-eared bats Myotis myotis and M. blythii. J. Exp. Biol. 210, 166-176. [DOI] [PubMed] [Google Scholar]
- Schmidt, S., Silja, H. and Jurgen, P. (2000). The role of echolocation in the hunting of terrestrial prey-new evidence for an underestimated strategy in the gleaning bat, Megaderma lyra. J. Comp. Physiol. A 186, 975-988. [DOI] [PubMed] [Google Scholar]
- Sontag, C. (1971). Spectral sensitivity studies on visual system of praying mantis, Tenodera sinensis. J. Gen. Physiol. 57, 93-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surlykke, A. and Moss, C. F. (2000). Echolocation behavior of big brown bats, Eptesicus fuscus, in the field and the laboratory. J. Acoust. Soc. Am. 108, 2419-2429. [DOI] [PubMed] [Google Scholar]
- Thompson, S. P. (1919). Calculus Made Easy. London: Macmillan.
- Triblehorn, J. D. and Yager, D. D. (2005a). Acoustic interactions between insects and bats: a model for the interplay of neural and ecological specializations. In Ecology of Predator-Prey Interactions, pp. 77-104. New York: Oxford University Press.
- Triblehorn, J. D. and Yager, D. D. (2005b). Timing of praying mantis evasive responses during simulated bat attack. J. Exp. Biol. 208, 1867-1876. [DOI] [PubMed] [Google Scholar]
- Triblehorn, J. D., Ghose, K., Bohn, K., Moss, C. F. and Yager, D. D. (2008). Free-flight encounters between praying mantids (V̇CO2) and bats (Eptesicus fuscus). J. Exp. Biol. 211, 555-562. [DOI] [PubMed] [Google Scholar]
- Yager, D. D. (1999). Structure, development, and evolution of insect auditory systems. Microsc. Res. Tech. 47, 380-400. [DOI] [PubMed] [Google Scholar]
- Yager, D. D., May, M. L. and Fenton, M. B. (1990). Ultrasound-triggered, flight-gated evasive maneuvers in the praying mantis Parasphendale agrionina. 1. Free flight. J. Exp. Biol. 152, 17-39. [DOI] [PubMed] [Google Scholar]
- Yuan, L. (1948). Homing and navigational courses of automatic target-seeking devices. J. Appl. Phys. 19, 1122-1128. [Google Scholar]