The disorders
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an arrhythmogenic cardiac disorder caused, in part, by dominant mutations in RYR2, the cardiac ryanodine receptor (RyR2) gene (Liu et al. 2009). The disorder manifests as syncopal events and sudden cardiac death, usually in response to intensive exercise; elevated catecholamines induce arrhythmogenic episodes that respond to β-blockers.
In other CPVT families arrhythmia is caused by recessive mutations in CASQ2 that cause truncation and/or misfolding of cardiac calsequestrin (CASQ2). In a mouse model, casq2-null or partially null mice also exhibit arrhythmias (Knollmann et al. 2006; Chopra et al. 2007; Knollmann, 2009, this symposium).
Malignant hyperthermia (MH) is an acute response to volatile anaesthetics, such as halothane, and depolarizing muscle relaxants, characterized by hypermetabolism, skeletal muscle contracture and fever (Loke & MacLennan, 1998) and caused, in part, by dominant mutations in RYR1. MH episodes can be reversed by dantrolene, an RyR1 Ca2+ release channel blocker. RyR1 MH mutant channels are activated by low levels of caffeine or halothane (Tong et al. 1999).
As a corollary of CPVT, ablation of casq1 causes MH (Dainese et al. 2009; Protasi et al. 2009, this symposium). CASQ1 mutations have not yet been reported to cause human MH.
Characteristics of RyR2 mutants in CPVT
It is accepted that CPVT arrhythmias result from repetitive, premature activation of RyR2. It has long been known that RyR is activated by elevation of luminal Ca2+ beyond a certain threshold (Palade et al. 1983; Venetucci et al. 2008). Here, we refer to this phenomenon as store overload-induced Ca2+ release (SOICR). RyR2 CPVT mutant channels expressed in the human embryonic kidney cell line HEK-293 were activated by much lower luminal Ca2+ levels than wild-type (wt) (Jiang et al. 2004, 2005). Critically, the frequency of Ca2+ oscillations was markedly increased and the size of the Ca2+ store was decreased. When single channel activities of RyR2 CPVT mutants were measured in planar lipid bilayers with 45 nm cytoplasmic Ca2+, incremental elevation of luminal Ca2+ from 0 to 1000 μm increased the probability of opening to 0.3–0.4 for CPVT mutant RyR2 vs. 0.02 for wt RyR2. These results demonstrate that RyR2 CPVT mutant channels, in isolation from other sarcoplasmic reticulum (SR) proteins, manifest a lower SOICR threshold than wt RyR2.
Characteristics of RyR1 mutants in MH
In comparable studies, RyR1 MH mutant channels expressed in HEK-293 cells are activated by much lower luminal Ca2+ levels than wt (Jiang et al. 2008). Furthermore, in single channel measurements, the MH-triggering agent, halothane, enhanced the frequency of activation of RyR1 MH mutant channels in the presence of luminal Ca2+, but had no effect in the absence of luminal Ca2+. Appropriately, dantrolene suppressed SOICR in HEK-293 cells expressing an RyR1 MH mutant. These results demonstrate that RyR1 MH mutations lower the threshold for luminal Ca2+ activation, that volatile anaesthetics further lower the SOICR threshold, and that dantrolene suppresses SOICR.
Characteristics of CASQ mutants in CPVT and MH
Calsequestrin plays at least three prominent roles: (1) it increases the SR luminal total Ca2+ content through its highly cooperative, high capacity, low affinity Ca2+ binding; (2) it buffers free Ca2+ levels in the SR lumen; and (3) it may convey bound Ca2+ into the RyR pore through its strategic localization in relation to triadin (Tri) and RyR (Wang et al. 1998; MacLennan & Reithmeier, 1998; Park et al. 2003; Royer & Ríos, 2009, this symposium). Mutations in CASQ can reduce that portion of the total Ca2+ binding and buffering capacity of the lumen that can be ascribed to CASQ in two ways: (1) through complete or partial ablation, as in casq-null mice or (2) through point or truncation mutations that affect key residues involved in such mechanisms as ‘domain swapping’ required for proper CASQ oligomerization, leading to cooperative, high capacity Ca2+ binding (Kim et al. 2007).
Common features of CPVT and MH
First, CPVT- and MH-susceptible individuals do not display abnormalities in muscle structure or function at rest. A CPVT episode is triggered in response to physical or emotional stress. A fulminant MH episode is triggered in response to halothane, stress or elevated temperature. As in arrhythmias, skeletal muscle contracture is a manifestation of abnormal, repetitive Ca2+ cycling. Second, causal RYR mutations are dominant, whereas causal CASQ mutations are recessive.
A unifying theory identifying SOICR as the common triggering mechanism for CPVT and MH episodes arising from mutations in RYR and CASQ genes
We propose that the aetiology triggering the arrhythmias caused by RYR2 or CASQ2 CPVT mutations or the muscle contracture caused by RYR1 or CASQ1 MH mutations is the same – the free SR luminal Ca2+ level repetitively exceeds the threshold for SOICR (Fig. 1). In CPVT, RyR2 mutants have a lower threshold for SOICR so that enhanced Ca2+ uptake, induced by β-adrenergic stimulation of the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), causes the level of free Ca2+ to overshoot its lowered SOICR threshold. CASQ2 mutants have reduced Ca2+ binding and buffering capacity, allowing free luminal Ca2+ levels to overshoot the normal RyR2 SOICR threshold when SERCA-dependent Ca2+ uptake is increased. In either case, repetitive SOICR manifests as arrhythmia.
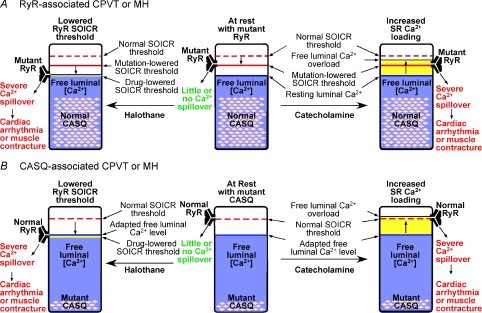
Figure 1. A model unifying the aetiology of CPVT and MH arising from mutations in RYR or CASQ genes.
The different thresholds for SOICR and the free SR luminal Ca2+ levels in CPVT or MH, associated with mutations in RYR (A) or CASQ genes (B), are illustrated in the resting state (middle panels) and stimulated states (left and right panels). The normal SOICR threshold is depicted as a dashed red bar. The SOICR threshold that is lowered as a consequence of RyR mutations, is depicted as a continuous red bar. The SOICR threshold that is lowered by drugs (halothane or caffeine) is depicted as a yellow bar in the left panels. The SR free luminal Ca2+ level is represented as a blue area. The pink lozenges within the blue areas represent functional Ca2+–CASQ complexes, which are reduced as a result of point or truncation mutants (as depicted) or abolished as a result of null mutations (not depicted). The yellow areas above the blue areas in the right panels represent an elevation, even if only transient, in the free Ca2+ levels, which, we propose, will occur when SERCA activity is enhanced by catecholamines. When the SOICR threshold is lowered below the SR free luminal Ca2+ level (left panels), or when the SR free luminal Ca2+ level surpasses, even transiently, the normal SOICR threshold (right panels), SOICR occurs, leading to a spillover of SR Ca2+ that can trigger spontaneous Ca2+ release, cardiac arrhythmias or skeletal muscle contracture.
In MH, RyR1 mutants have a lowered threshold for SOICR. In the presence of halothane, which further lowers its mutation-lowered SOICR threshold, RyR1 will be triggered to open when the free SR luminal Ca2+ level overshoots its lowered SOICR threshold. CASQ1 mutants have a reduced Ca2+ binding and buffering capacity, allowing free luminal Ca2+ levels to overshoot the halothane-lowered SOICR threshold of wt RyR1. In either case, a cycle is initiated in which SERCA pumps only partially refill the luminal Ca2+ store before SOICR is triggered. Repetitive SOICR elevates cytosolic Ca2+ to provide the signal for skeletal muscle contracture and hypermetabolism, whereas fever arises from enhanced ATP hydrolysis by SERCA and myosin.
An alternative proposal for the relationship between RyR and CASQ in CPVT and MH
The unifying theory proposed above has a simplicity not found in an alternate explanation of the aetiology of CPVT and MH. Experiments by Györke & Terentyev (2008; Györke et al. 2009, this symposium) and Qin et al. (2008) lead them to postulate that a CASQ2–Tri–RyR2 complex regulates RyR2 function and to assign a 4th and even a 5th role to CASQ in this complex: (4) the deactivation of RyR in low luminal Ca2+; and (5) the activation of RyR by elevated luminal Ca2+. Their theory is that mutations in CASQ2 (and, by extension, mutations in CASQ1) alter protein–protein interactions within the complex, resulting in the abnormal openings of RyR2 (or RyR1) that cause episodes of CPVT (or MH). There is general agreement in both theories and for both disorders, whether caused by mutations in RyR or CASQ, that manifestations of disease are ultimately caused by the abnormal response of RyR channels to elevations in luminal Ca2+.
To evaluate the relative merits of the hypotheses that SOICR acts directly through RyR or through a RyR–Tri–CASQ complex, let us consider critical features of the diseases. First, the dominant inheritance of RyR mutations in CPVT and MH is expected, since openings of abnormal channels in the presence of normal closed channels can cause full disease manifestations. Why then are all cases of CPVT caused by CASQ2 mutations inherited recessively? Surely if dysregulation of a CASQ2–Tri–RyR2 complex were causal, then mutation of one CASQ2 allele would be sufficient to trigger abnormal activation in abnormal complexes, manifesting as an arrhythmia – but this is not the case.
Second, CASQ2 CPVT causal mutations have diverse properties: R33Q activates wt RYR2 channels in response to elevated luminal Ca2+, whereas L167H did not (Qin et al. 2008); R33Q is an unstable molecule and may be degraded (Rizzi et al. 2008); R33Q causes a compensatory elevation of the expression of RyR2 and calreticulin (Song et al. 2007); R33Q, but not CASQdel lowers both basal and nadir SR Ca2+ levels (Terentyev et al. 2008). In contrast to those papers that relate differences among CASQ2 mutants to CPVT, one paper pinpoints the common feature of all CASQ2 CPVT mutants – their loss of the ability to oligomerize properly and therefore to bring about cooperative high capacity Ca2+ binding (Kim et al. 2007). Thus, the critical common feature in CASQ2-associated CPVT is a decrease in luminal Ca2+ binding and Ca2+ buffering that would permit an overshoot of luminal Ca2+ when SERCA pumps are stimulated.
Final questions are: why, if CASQ is critical to the regulation of normal RyR channels, do CASQ2-null humans or casq2-null mice, susceptible to CPVT, only display arrhythmias under stressful conditions that elevate luminal Ca2+ loading; and why do casq1-null mice only display MH symptoms in the presence of MH-triggering anaesthetics or heat? The answers cannot lie in CASQ–Tri–RyR interactions, since CASQ is absent. Therefore, the answer is most likely to lie in normal RyR itself, which, we propose, is intrinsically programmed to open in response to a tsunami of Ca2+ in a luminal space depleted of its capacity to buffer a surge of incoming Ca2+ (CPVT), or to open in response to halothane, which lowers its SOICR threshold (MH).
Acknowledgments
This work was supported by Grants to D.H.M. from the Canadian Institutes of Health Research (CIHR) and to S.R.W.C. from the National Institutes of Health (HL75210) and CIHR and the Alberta Heritage Foundation for Medical Research.
References
- Chopra N, Kannankeril PJ, Yang T, Hlaing T, Holinstat I, Ettensohn K, Pfeifer K, Akin B, Jones LR, Franzini-Armstrong C, Knollmann BC. Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ Res. 2007;101:617–626. doi: 10.1161/CIRCRESAHA.107.157552. [DOI] [PubMed] [Google Scholar]
- Dainese M, Quarta M, Lyfenko AD, Paolini C, Canato M, Reggiani C, Dirksen RT, Protasi F. Anesthetic- and heat-induced sudden death in calsequestrin-1-knockout mice. FASEB J. 2009 doi: 10.1096/fj.08-121335. DOI 10.1096/fj.08-121335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S, Stevens SCW, Terentyev D. Cardiac calsequestrin: quest inside the SR. J Physiol. 2009;587:3091–3094. doi: 10.1113/jphysiol.2009.172049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008;77:245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- Jiang D, Chen W, Xiao J, Wang R, Kong H, Jones PP, Zhang L, Fruen B, Chen SR. Reduced threshold for luminal Ca2+ activation of RyR1 underlies a causal mechanism of porcine malignant hyperthermia. J Biol Chem. 2008;283:20813–20820. doi: 10.1074/jbc.M801944200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, Zhang L, Chen SRW. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SRW. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci U S A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Youn B, Kemper L, Campbell C, Milting H, Varsanyi M, Kang C. Characterization of human cardiac calsequestrin and its deleterious mutants. J Mol Biol. 2007;373:1047–1057. doi: 10.1016/j.jmb.2007.08.055. [DOI] [PubMed] [Google Scholar]
- Knollmann BC. New roles of calsequestrin and triadin in cardiac muscle. J Physiol. 2009;587:3081–3087. doi: 10.1113/jphysiol.2009.172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Rizzi N, Boveri L, Priori SG. Ryanodine receptor and calsequestrin in arrhythmogenesis: What we have learnt from genetic diseases and transgenic mice. J Mol Cell Cardiol. 2009;46:149–159. doi: 10.1016/j.yjmcc.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Loke JCP, MacLennan DH. Malignant hyperthermia and central core disease: disorders of Ca2+ release channels. Am J Med. 1998;104:470–486. doi: 10.1016/s0002-9343(98)00108-9. [DOI] [PubMed] [Google Scholar]
- MacLennan DH, Reithmeier RA. Ion tamers. Nat Struct Biol. 1998;5:409–411. doi: 10.1038/nsb0698-409. [DOI] [PubMed] [Google Scholar]
- Palade P, Mitchell RD, Fleischer S. Spontaneous calcium release from sarcoplasmic reticulum. General description and effects of calcium. J Biol Chem. 1983;258:8098–8107. [PubMed] [Google Scholar]
- Park H, Wu S, Dunker AK, Kang C. Polymerization of calsequestrin. Implications for Ca2+ regulation. J Biol Chem. 2003;278:16176–16182. doi: 10.1074/jbc.M300120200. [DOI] [PubMed] [Google Scholar]
- Protasi F, Paolini C, Dainese M. Calsequestrin-1: a new candidate gene for malignant hyperthermia and exertional/environmental heat stroke. J Physiol. 2009;587:3095–3100. doi: 10.1113/jphysiol.2009.171967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Valle G, Nani A, Nori A, Rizzi N, Priori SG, Volpe P, Fill M. Luminal Ca2+ regulation of single cardiac ryanodine receptors: insights provided by calsequestrin and its mutants. J Gen Physiol. 2008;131:325–334. doi: 10.1085/jgp.200709907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi N, Liu N, Napolitano C, Nori A, Turcato F, Colombi B, Bicciato S, Arcelli D, Spedito A, Scelsi M, Villani L, Esposito G, Boncompagni S, Protasi F, Volpe P, Priori SG. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model. Circ Res. 2008;103:298–306. doi: 10.1161/CIRCRESAHA.108.171660. [DOI] [PubMed] [Google Scholar]
- Royer L, Ríos R. Deconstructing calsequestrin. Complex buffering in the calcium store of skeletal muscle. J Physiol. 2009;587:3101–3111. doi: 10.1113/jphysiol.2009.171934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, Berul CI, Eldar M, Seidman CE, Seidman JG. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2007;117:1814–1823. doi: 10.1172/JCI31080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Kubalova Z, Valle G, Nori A, Vedamoorthyrao S, Terentyeva R, Viatchenko-Karpinski S, Bers DM, Williams SC, Volpe P, Gyorke S. Modulation of SR Ca release by luminal Ca and calsequestrin in cardiac myocytes: effects of CASQ2 mutations linked to sudden cardiac death. Biophys J. 2008;95:2037–2048. doi: 10.1529/biophysj.107.128249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, McCarthy TV, MacLennan DH. Measurement of resting cytosolic Ca2+ concentrations and Ca2+ stores in HEK-293 cells transfected with malignant hyperthermia or central core disease mutant Ca2+ release channels. J Biol Chem. 1999;274:693–702. doi: 10.1074/jbc.274.2.693. [DOI] [PubMed] [Google Scholar]
- Venetucci LA, Trafford AW, O’Neill SC, Eisner DA. The sarcoplasmic reticulum and arrhythmogenic calcium release. Cardiovasc Res. 2008;77:285–292. doi: 10.1093/cvr/cvm009. [DOI] [PubMed] [Google Scholar]
- Wang S, Trumble WR, Liao H, Wesson CR, Dunker AK, Kang CH. Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat Struct Biol. 1998;5:476–483. doi: 10.1038/nsb0698-476. [DOI] [PubMed] [Google Scholar]