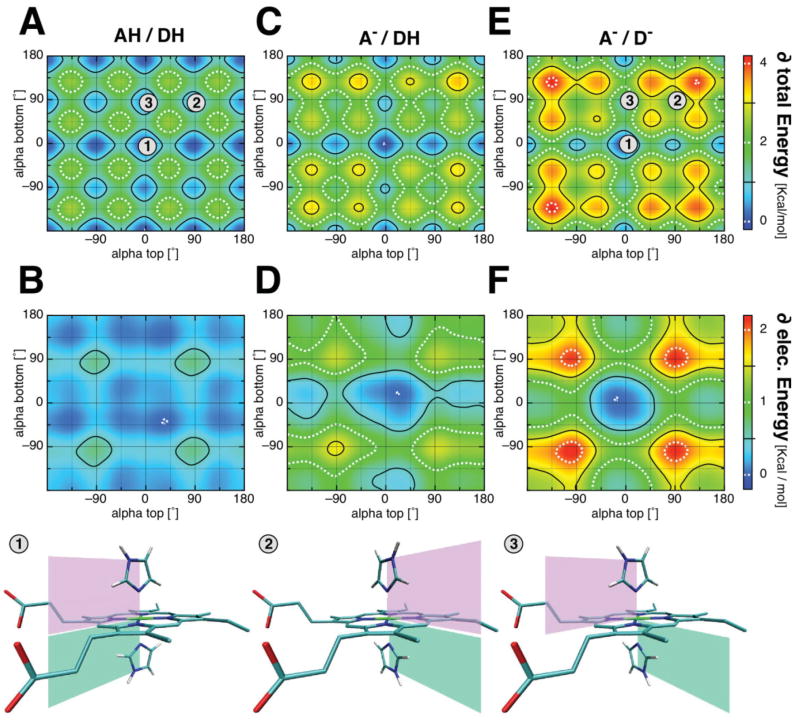
Figure 5.
Dependence of the interaction energy on the histidine orientation and propionic acid ionization state for a bis-imidazole heme model complex. Interaction energies between imidazole ligands and the heme were calculated with MCCE at ε = 6. Shown are the total interaction energy (top row) and its electrostatic contribution (middle row); (A, B) when both propionates are protonated (AH, DH); (C, D) when the propionatic acid attached to ring A is deprotonated (A−, DH) and, (E, F) when both propionates are deprotonated (A−, D−). The change in the energy landscape with ligand orientation, alpha is illustrated (see Table II for absolute values). Bottom row shows three ligand conformations marked in the total energy plots with the numbers 1–3.