Abstract
Purpose
We examined the activity of bortezomib, dexamethasone, and rituximab (BDR) in patients with symptomatic, untreated Waldenström macroglobulinemia (WM).
Patients and Methods
A cycle of therapy consisted of bortezomib 1.3 mg/m2 intravenously; dexamethasone 40 mg on days 1, 4, 8, and 11; and rituximab 375 mg/m2 on day 11. Patients received four consecutive cycles for induction therapy and then four more cycles, each given 3 months apart, for maintenance therapy. Twenty-three patients received a median of seven cycles of treatment.
Results
Median bone marrow disease involvement declined from 55% to 10% (P = .0004), serum immunoglobulin M levels declined from 4,830 to 1,115 mg/dL (P < .0001), and hematocrit increased from 29.8% to 38.2% (P = .0002) at best response. The overall response rates and major response rates were 96% and 83% with three complete responses, two near complete responses, three very good partial responses, 11 partial responses, and three minor responses. Responses occurred at a median of 1.4 months. With a median follow-up of 22.8 months, 18 of 23 patients remained free of disease progression. Peripheral neuropathy was the most common toxicity, and it resolved to grade ≤ 1 in 13 of 16 patients at a median of 6.0 months. Four of the first seven treated patients developed herpes zoster, resulting in the institution of prophylactic antiviral therapy.
Conclusion
The results demonstrate that BDR produces rapid and durable responses, along with high rates of response and complete remissions in WM. Herpes zoster prophylaxis is necessary with BDR, and reversible peripheral neuropathy was the most common toxicity leading to premature discontinuation of bortezomib in 61% of patients. Exploration of alternative schedules for bortezomib administration that includes weekly dosing should be pursued.
INTRODUCTION
Waldenström macroglobulinemia (WM) is a B-cell disorder, which is characterized by bone marrow infiltration with lymphoplasmacytic cells and an immunoglobulin M (IgM) monoclonal gammopathy.1–3 Familial predilection has commonly been observed in WM.4 Despite advances in therapy, WM remains incurable, and novel therapeutic agents are urgently needed.
Bortezomib is a proteasome inhibitor that shows potent in vitro activity against primary WM cells and cell lines,5–7 as well as significant single-agent activity in the salvage therapy of patients with WM in whom overall response rates (ORRs) of 60% to 80% and major response rates of 50% to 60% have been observed.8–11 No complete responses (CRs) were observed in these series, and the median time to progression (TTP) was 8 to 9 months. Rituximab has been an important mainstay in the therapy of patients with WM, having ORRs of 27% to 35% and median durations of response of 8 to 27 months when used as monotherapy.12–15 More recently, the use of an extended treatment schedule for rituximab has been evaluated in patients who received eight infusions of rituximab (375 mg/m2/wk) at weeks 1 to 4 and 12 to 16. ORRs of 44% to 48% were observed in these studies, with median durations of response of 16 to 29 months.16,17 No CRs have been observed in these series.
Although the importance of attaining a CR/near complete response (nCR) has been reported as a predictive variable for progression-free survival in multiple myeloma,18–21 a recent study by the Waldenström's Macroglobulinemia Clinical Trials Group (WMCTG) showed that attainment of at least a very good partial response (VGPR) (> 90% reduction in IgM) predicted a significantly longer TTP.22 However, the attainment of CR/nCR has been elusive in most clinical trials involving WM patients in whom CR/nCR rates of 5% to 10% have been observed with various combination therapies, including rituximab-based therapies.22–26 Moreover, concerns about the impact of therapy on stem cell collection and long-term safety have been raised in several recent publications, invoking a greater need to explore novel treatment strategies in WM.22,27,28
In view of these considerations, we and others have sought to develop more effective upfront strategies for WM therapy, while avoiding the use of oral alkylator and nucleoside analog agents. In preclinical studies, additive and possibly synergistic tumor cell killing have been demonstrated in various lymphoma and myeloma models with various combinations of bortezomib, rituximab, and/or corticosteroids.29–31 Thus, we pursued the clinical exploration of bortezomib, dexamethasone, and rituximab (BDR) in the upfront treatment of patients with WM.
PATIENTS AND METHODS
WM patients requiring therapy based on consensus recommendations32 who were previously untreated and who had a platelet count of ≥ 50 × 109/L, absolute neutrophil count of ≥ 0.75 × 109/L, creatinine clearance of ≥ 30 mL/min, total serum bilirubin of less than 2.0, AST/ALT of less than 3× the upper limit of normal, and a Karnofsky performance status ≥ 60% were eligible. Patients who were pregnant or lactating, had serious comorbid disease, or had any uncontrolled infection or active second malignancy were not eligible. Patients with peripheral neuropathy (PN) were not excluded from participation in this study. Contraception was required for all patients of reproductive potential for the duration of protocol treatment.
All patients provided informed written consent, and the institutional review boards of all participating institutions granted study approval. Intended therapy consisted of four continuous cycles of BDR followed by a 12-week pause and then four additional cycles of BDR spaced 12 weeks apart. This schedule was chosen in hopes of attenuating the development of PN, because in our earlier experience with bortezomib monotherapy, the incidence of PN increased after four continuous cycles of bortezomib therapy. In this study, a cycle of therapy consisted of bortezomib 1.3 mg/m2 intravenously (IV); dexamethasone 40 mg IV days 1, 4, 8, and 11; and rituximab 375 mg/m2 IV on day 11. Dose modification and delay of therapy were permitted. All toxicities with the exception of PN were managed as follows: For any ≥ grade 3 nonhematologic toxicity or grade 4 hematologic toxicity, the offending study drug was determined and held up to 2 weeks. For nonhematologic toxicity, the offending study drug was held until toxicity returned to ≤ grade 1. For hematologic toxicities, the offending drug was held until the patient had hemoglobin ≥ 7.5 g/dL, absolute neutrophil count ≥ 0.5 × 109/L, and platelet count ≥ 30 × 109/L. The use of transfusions or cytokine support to meet retreatment criteria was permitted. Treatment interruption was not required for lymphopenia. If the toxicity did not resolve during the 2-week hold period as noted, the offending study drug was discontinued. If the toxicity resolved during the 2-week hold period, restart at the same dose level was permitted. If the toxicity recurred, therapy was again held as above. Restart for bortezomib was permitted but with a dose reduction to 1.0 mg/m2 after the second hold period and to 0.7 mg/m2 after a third hold period for recurring toxicity. Bortezomib was permanently discontinued if a fourth hold period was required. Patients experiencing bortezomib-related PN had their dose reduced using guidelines previously established in patients with multiple myeloma.33,34 Dose reduction of rituximab or dexamethasone was not permitted, but either drug could be held for toxicities as noted above. The use of diphenhydramine, acetaminophen and, at the treating physician's discretion, corticosteroids and/or ranitidine or cimetidine was permitted for rituximab infusion prophylaxis. Granulocyte colony-stimulating factor, erythropoietin, and transfusions of packed RBCs or platelets were permitted to support patient's counts during therapy.
The study was amended to strongly recommend herpes zoster prophylaxis after four of the first seven patients developed shingles. Valcyclovir 1 g once a day or acyclovir 400 mg twice a day was recommended for shingles prophylaxis. In addition, the prophylactic use of plasmapheresis was recommended for patients demonstrating an IgM level of ≥ 5,000 mg/dL before the administration of rituximab, given the potential for rituximab-mediated IgM flare and aggravation of hyperviscosity.16,35,36
Patients were assessed at each cycle and were eligible for continuation of therapy in the absence of progressive disease. Patients were also assessed for ≤ 30 days after completion of the first four cycles of induction therapy and the last four cycles of maintenance therapy. Baseline studies consisted of CBCs and differential; quantitative serum IgM levels; serum protein electrophoresis; bone marrow biopsy and aspiration; computed tomography scans of the chest, abdomen, and pelvis; serum electrolytes; liver function tests; blood urea nitrogen; creatinine; and serum β2-microglobulin levels. Restaging studies included CBC and differential; quantitative serum IgM levels; serum protein electrophoresis; a bone marrow biopsy and aspiration (to confirm CR); and computed tomography scans of the chest, abdomen, and pelvis if extramedullary disease was present at baseline. Response determinations were made using modified consensus panel criteria from the Third International Workshop on Waldenström's Macroglobulinemia,37 and response rates were determined on an intent-to-treat basis. A CR was defined as having resolution of all symptoms, normalization of serum IgM levels with complete disappearance of IgM paraprotein by immunofixation, absence of bone marrow disease by bone marrow biopsy and aspiration, and resolution of any adenopathy or splenomegaly. An nCR was defined as fulfilling all CR criteria in the presence of a positive immunofixation study. Patients with VGPR, partial response (PR), and minor response were defined as having a ≥ 90%, ≥ 50%, and 25% to 49% reduction in serum IgM levels, respectively. Progressive disease occurred when a more than 25% increase in serum IgM level or progression of clinically significant disease parameters was observed. TTP was calculated from the start of therapy using the Kaplan-Meier method. The primary end points of this study were overall response, median progression-free survival, and toxicity.
Statistical analysis included comparison of pre- and post-treatment parameters performed using a two-tailed t test in Excel software (Microsoft, Redmond, WA). For nonparametric testing of pre- and post-treatment responses, two-tailed Fisher's exact test (VassarStats; Vassar College, Poughkeepsie, NY) was used. A P value ≤ .05 was deemed to be significant for these studies.
RESULTS
Patient and Disease Characteristics
Twenty-three of the intended 25 patients were enrolled onto this study, which was ended prematurely in favor of a successor study that used a once-a-week bortezomib schedule. The baseline characteristics for the patients on this study are listed in Table 1. Treatment initiation was based on consensus criteria32 and included anemia (n = 16), hyperviscosity (n = 5), and symptomatic amyloidosis (n = 2). Of the 23 patients enrolled onto the study, 19 received treatment beyond four cycles of therapy, and 18 patients completed all eight cycles. Three patients were removed after completing four cycles of induction therapy because of lack of response (n = 1) and progressive disease (n = 2), whereas two patients elected not to continue with therapy (one after cycle 4 of induction, one after cycle 5), both of whom were in a major response (one CR, one PR).
Table 1.
Baseline Characteristics for All Patients Enrolled Onto Study
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 66 | |
| Range | 48-86 | |
| No prior therapy | 23 | 100 |
| Bone marrow involvement | ||
| Median | 55 | |
| Range | 5-90 | |
| Adenopathy and/or splenomegaly | 4 | 17 |
| IgM, mg/dL | ||
| Median | 4,830 | |
| Range | 458-9,950 | |
| IgM ≥ 3,000 mg/dL | 13 | 57 |
| Hematocrit, % | ||
| Median | 28.9 | |
| Range | 19.5-37 | |
| Hematocrit ≤ 30% | 13 | 57 |
| Platelets ≤ 100,000/μL | 2 | 9 |
| β2-microglobulin, mg/L | ||
| Median | 3.3 | |
| Range | 1.0-7.1 | |
Abbreviation: IgM, immunoglobulin M.
Response
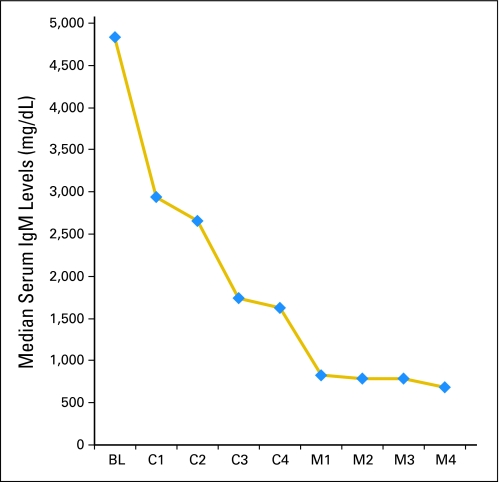
Median IgM levels for all 23 patients declined from 4,830 mg/dL (range, 458 to 9,950 mg/dL) pretherapy to 1,115 mg/dL (range, 18 to 4,930 mg/dL) at best response (P < .0001). Pretherapy, 13 of 23 patients (57%) demonstrated an IgM level ≥ 3,000 mg/dL; after treatment, only two of 23 (9%) had an IgM level ≥ 3,000 mg/dL (P = .0012 by Fisher's exact t test). Median serum IgM levels after each cycle of induction (C1 to C4) and maintenance (M1 to M4) therapy are shown in Figure 1. Bone marrow involvement also decreased after therapy, with a decline in the median percentage of tumor cell involvement from 55% (range, 5% to 90%) to 10% (range, 0% to 70%); P = .0004. Categorical responses were as follows: CR, n = 3; nCR, n = 2; VGPR, n = 3; PR, n = 11; and minor response, n = 3 for ORRs and major response rates of 96% and 83%, respectively. Among responders, the median time to a ≥ 25% reduction in serum IgM was 1.4 months (range, 0.7 to 15 months), whereas the median time to best response for responding patients was > 15 months (range, 0.7 to > 26 months).
Fig 1.
Median serum immunoglobulin M (IgM) levels at baseline (BL), and after each cycle of induction (C1 to C4) and maintenance (M1 to M4) therapy in patients treated with bortezomib, dexamethasone, and rituximab.
TTP
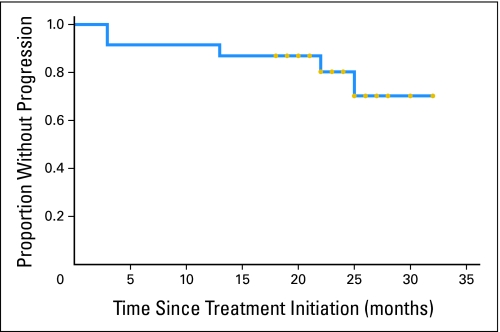
With a median follow-up of 22.8 months (range, 3.3 to 33.2 months), all patients are alive and 18 patients remain free of disease progression. Among the five patients who experienced disease progression, one had stable disease after four cycles of induction therapy, and four patients had a PR. The Kaplan-Meier curve for TTP for all patients is shown in Figure 2. Estimation for median TTP was not possible because of insufficient events, but it exceeds 30 months.
Fig 2.
Kaplan-Meier curve for time to progression for patients on study. Gold circles denote last follow-up for individual patients.
Changes in Hematologic Parameters in Treatment of WM Patients
A significant increase in the median hematocrit was noted for all patients from 29.8% (range, 19.5% to 50.5%) before therapy to 38.2% (range, 28% to 47.8%) after treatment (P < .00001), with 16 (70%) of 23 patients demonstrating an absolute increase in hematocrit of more than 2%. Before therapy, the median platelet count was 198 × 109/L (range, 91 to 545 × 109/L) to 235 × 109/L (range, 80 to 385 × 109/L); P = .94. Pretherapy, 13 (57%) and two (9%) of the 23 patients demonstrated a hematocrit ≤ 30% and a platelet count of ≤ 100 × 109/L, respectively. After therapy, two (9%) and one (4.3%) of the 23 patients demonstrated a hematocrit of ≤ 30% and platelet count of ≤ 100 × 109/L (P = .0012 and P = 1.0, respectively).
Toxicities
Toxicities of grade ≥ 2 encountered in this study are listed in Table 2. PN was the most common toxicity encountered in this study, and it resulted in premature discontinuation of bortezomib in 14 patients (61%). Discontinuation of bortezomib occurred after a median of four cycles (range, three to six cycles) of treatment in these patients. In three patients, dexamethasone was discontinued because of persistent hyperglycemia (n = 1), GI intolerance in the absence of bleeding (n = 1), and corticosteroid-related myopathy (n = 1) at cycles 3, 5, and 6, respectively. In one patient, rituximab was discontinued at cycle 7 because of suspected rituximab-related late neutropenia. Toxicities of grade ≥ 2 are listed in Table 2. Among the 16 patients experiencing grade ≥ 2 PN, resolution to grade ≤ 1 occurred in 13 patients (81%) at a median time of 6.0 months (range, 1.9 to 29.9 months) after onset of the PN.
Table 2.
Adverse Events Possibly, Probably, or Definitely Associated With Protocol Therapy
| Toxicity Type | Grade 2 |
Grade 3 |
Grade 4 |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Anemia | 18 | 78 | 1 | 4 | 0 | 0 |
| Anorexia | 2 | 9 | 0 | 0 | 0 | 0 |
| Arrythmia | 2 | 9 | 1 | 4 | 0 | 0 |
| Cough | 3 | 13 | 0 | 0 | 0 | 0 |
| Diarrhea | 2 | 9 | 0 | 0 | 0 | 0 |
| Dehydration | 3 | 13 | 0 | 0 | 0 | 0 |
| Dyspnea | 3 | 13 | 0 | 0 | 0 | 0 |
| Edema | 1 | 4 | 0 | 0 | 0 | 0 |
| Fatigue | 4 | 17 | 0 | 0 | 0 | 0 |
| Herpes zoster | 5 | 22 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 6 | 26 | 0 | 0 | 0 | 0 |
| Hypotension | 0 | 0 | 1 | 4 | 0 | 0 |
| Insomnia | 3 | 13 | 0 | 0 | 0 | 0 |
| Infection without neutropenia | 10 | 43 | 0 | 0 | 0 | 0 |
| Infection with neutropenia | 0 | 0 | 1 | 4 | 0 | 0 |
| Memory impairment | 1 | 4 | 0 | 0 | 0 | 0 |
| Myopathy | 0 | 0 | 1 | 4 | 0 | 0 |
| Neutropenia | 6 | 26 | 6 | 26 | 1 | 4 |
| Pneumonia | 2 | 9 | 1 | 4 | 0 | 0 |
| Peripheral neuropathy | 9 | 39 | 7 | 30 | 0 | 0 |
| Rash | 1 | 4 | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 8 | 35 | 2 | 9 | 0 | 0 |
Rituximab-Induced Hyperviscosity
Plasmapheresis was strongly recommended for patients who had a pretherapy serum viscosity of ≥ 3.5 centipoise or serum IgM of more than 5,000 mg/dL to avoid rituximab-induced symptomatic IgM flare.16,35,36 Six patients therefore underwent prophylactic pretherapy plasmapheresis. A spike in serum IgM level (≥ 25%) associated with rituximab at any point during therapy was observed in 2 of 23 patients (9%), both of whom required plasmapheresis.
DISCUSSION
We examined the combination of BDR as a novel regimen for primary therapy of WM, given preclinical studies supporting potential synergism of these agents.29–31 The outcome of this study demonstrated an ORR of 96%, including an 83% major response rate. Importantly, 22% of the patients achieved a CR/nCR and 35% achieved a VGPR or better. These responses compare favorably with those achieved by one of the agents alone (ie, bortezomib, rituximab, or dexamethasone), for which ORR and major response rates of 40% to 50% have been reported. Importantly, no CRs/nCRs have been observed with either bortezomib or rituximab alone in the primary treatment setting.8–10,15–17 Furthermore, the CR/nCR rate observed in this study seems higher than those previously observed with other rituximab-based regimens, in which CR/nCR rates of 0% to 10% have been reported.23–26,38,39 The attainment of VGPR or better may be an important prognostic marker for progression-free survival, as several myeloma studies and at least one long-term outcomes study in WM have suggested.18–21 Notably, no patient attaining a VGPR or better has so far demonstrated progression.
An important consideration in this series was the rapid time to response. The median time to a ≥ 25% reduction in serum IgM in this series was 1.4 months, which compares favorably with most of the monotherapies currently in use as well as combination therapies with rituximab with times to response of more than 3 to 4 months16,17,22-26,39 Therefore, in WM patients in whom more rapid responses may be required (eg, those patients with symptomatic hyperviscosity, cryoglobulinemia, or autoimmune cytopenias), the use of BDR may represent a particularly attractive treatment option. Also important in the consideration of treatment choice is the potential impact of the rituximab-induced IgM flare, which may lead to symptomatic hyperviscosity, worsening of IgM-related neuropathy, cryoglobulinemia, and other IgM-related autoimmune complications. In previous studies, we and others observed that bortezomib could have an impact on the secretion of IgM independent of WM tumor cell killing, suggesting a potentially suppressive role for bortezomib in blocking IgM release.8,40 As observed in this study, only two patients (9%) treated with BDR exhibited a rituximab-related IgM flare while receiving treatment, which compares favorably with 25% to 75% of patients experiencing flares with rituximab monotherapy or with other rituximab combinations, including cyclophosphamide, nucleoside analogs, thalidomide, and lenalidomide.16,17,25,35,36,38,40,41 The sequencing of rituximab (ie, delaying rituximab infusion to the end of the therapeutic cycle v using it at the beginning) seems to play an important role in mitigating the IgM flare with nucleoside analogs40 and may also account for the decreased incidence of IgM flare observed in this study, wherein rituximab was given on day 11 of each cycle after bortezomib and dexamethasone. Despite these findings, clinicians should consider prophylactic plasmapheresis before any rituximab administration in patients with elevated serum IgM or serum viscosity levels (ie, > 5,000 mg/dL and 3.5 CP, respectively). Following treatment, serum IgM and viscosity levels should be closely monitored to determine whether additional plasmapheresis may be required.
Although the median TTP has not been reached in this study, it appears to be more than 30 months compared with that previously reported for either rituximab (14 to 27 months) or bortezomib (16 months) alone when used as first-line therapy.9,15–17 In addition, the response duration with BDR seems to be at least on par with those previously reported with other rituximab-containing regimens, including those in combination with cyclophosphamide, nucleoside analogs, and thalidomide.22–26,39
The most notable toxicity encountered in this study was sensory PN, which was reversible in more than 80% of patients at a median of 6.0 months. The incidence and reversibility of bortezomib-related PN in this study were similar to those previously encountered in single-agent experiences with bortezomib in patients with relapsed refractory WM and in other patient populations. The incidence of grade 3 PN in these collective experiences suggests that WM patients may have a greater propensity for developing more severe bortezomib-related neuropathy, given that grade ≥ 3 neuropathy was observed in 20% to 30% of WM patients versus 2% to 12% in patients with follicular lymphoma, mantle cell lymphoma, and multiple myeloma.11,42–45 An increased propensity for WM patients to develop drug-induced PN has also been observed with other agents, including thalidomide and vincristine,25,38 and therefore more vigilant monitoring, better patient education, and earlier dose modification should be considered with all neuropathic agents, including bortezomib in patients with WM. In addition, the use of an alternative dose and schedule of bortezomib (ie, bortezomib 1.6 mg/m2 once a week) may represent a more neuropathy-sparing approach in WM patients, as suggested in ongoing studies by Ghobrial et al.46
The unexpected high incidence of herpes zoster, which occurred in four of the first seven patients treated during this study, prompted the routine use of herpes zoster prophylaxis with either valcyclovir or acyclovir with good effect. Only one patient who did not fill her prescription for valcyclovir subsequently developed herpes zoster. The incidence of herpes zoster came as a surprise because in our earlier experience with bortezomib monotherapy in WM, the incidence of herpes zoster was low (0% to 10%).8–11 Similarly, in myeloma patients treated in the APEX (Effects of Allopurinol on Coronary and Peripheral Endothelial Function in Patients With Cardiac Syndrome X) study with bortezomib alone, the incidence of herpes zoster was 13%.47 The use of corticosteroids/and or rituximab may confer an added risk for herpes zoster when used with bortezomib, and therefore herpes zoster prophylaxis should be used with BDR. The shingles prophylaxis vaccine (Zostavax; Merck, Whitehouse Station, NJ) should not be used in this regard because it is contraindicated in this patient population.
In summary, BDR produces rapid and durable responses, along with high rates of response and complete remissions in WM. The use of BDR offers a stem cell–sparing approach to the therapy of WM and may be a particularly suitable regimen for those patients in whom more rapid remissions are needed. Herpes zoster prophylaxis is necessary with BDR, and reversible PN is the most common toxicity observed on the twice-a-week schedule of bortezomib used in this study.
Footnotes
Supported by the Peter and Helen Bing Fund for Waldenström's Macroglobulinemia at the Dana-Farber Cancer Institute, Millennium: The Takeda Oncology Company, and National Institutes of Health Career Development Award No. K23CA087977-03 (S.P.T.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00250926.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Thomas J. Myers, Millennium: The Takeda Oncology Company (C); Andy Boral, Millennium: The Takeda Oncology Company (C); Ann Birner, Millennium: The Takeda Oncology Company (C); Dixie L. Esseltine, Millennium: The Takeda Oncology Company (C) Consultant or Advisory Role: Steven P. Treon, Millennium: The Takeda Oncology Company (C); Irene M. Ghobrial, Milleniun: The Takeda Oncology Company (C) Stock Ownership: Thomas J. Myers, Millennium: The Takeda Oncology Company; Andy Boral, Millennium: The Takeda Oncology Company; Ann Birner, Millennium: The Takeda Oncology Company; Dixie L. Esseltine, Millennium: The Takeda Oncology Company Honoraria: Steven P. Treon, Millennium: The Takeda Oncology Company; Irene M. Ghobrial, Millennium: The Takeda Oncology Company Research Funding: Steven P. Treon, Millennium: The Takeda Oncology Company; Irene M. Ghobrial, Millennium: The Takeda Oncology Company Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Steven P. Treon, Thomas J. Myers, Andy Boral, Dixie L. Esseltine
Administrative support: Leukothea Ioakimidis, Jacob D. Soumerai, Christopher J. Patterson
Provision of study materials or patients: Steven P. Treon, Patricia Sheehy, Marybeth Nelson, Michael Willen, Jeffrey Matous, John Mattern II, Jakow G. Diener, George P. Keogh, Irene M. Ghobrial
Collection and assembly of data: Steven P. Treon, Leukothea Ioakimidis, Jacob D. Soumerai, Christopher J. Patterson, Patricia Sheehy, Marybeth Nelson, Michael Willen, Jeffrey Matous, John Mattern II, Jakow G. Diener, George P. Keogh
Data analysis and interpretation: Steven P. Treon, Leukothea Ioakimidis, Jacob D. Soumerai, Christopher J. Patterson, Ann Birner, Dixie L. Esseltine, Irene M. Ghobrial
Manuscript writing: Steven P. Treon
Final approval of manuscript: Steven P. Treon, Leukothea Ioakimidis, Jacob D. Soumerai, Christopher J. Patterson, Patricia Sheehy, Marybeth Nelson, Michael Willen, Jeffrey Matous, John Mattern II, Jakow G. Diener, George P. Keogh, Thomas J. Myers, Andy Boral, Ann Birner, Dixie L. Esseltine, Irene M. Ghobrial
REFERENCES
- 1.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenström's macroglobulinemia: Consensus panel recommendations from the Second International Workshop on Waldenström's Macroglobulinemia. Semin Oncol. 2003;30:110–115. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 2.Harris NL, Jaffe ES, Stein H. A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 3.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 4.Treon SP, Hunter ZR, Aggarwal A, et al. Characterization of familial Waldenstrom's macroglobulinemia. Ann Oncol. 2006;17:488–494. doi: 10.1093/annonc/mdj111. [DOI] [PubMed] [Google Scholar]
- 5.Mitsiades CS, Mitsiades N, Richardson PG, et al. Novel biologically based therapies for Waldenstrom's macroglobulinemia. Semin Oncol. 2003;30:309–312. doi: 10.1053/sonc.2003.50065. [DOI] [PubMed] [Google Scholar]
- 6.Roccaro AM, Leleu X, Sacco A, et al. Dual targeting of the proteasome regulates survival and homing in Waldenstrom macroglobulinemia. Blood. 2008;111:4752–4763. doi: 10.1182/blood-2007-11-120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leleu X, Eechoute J, Jia X, et al. Targeting NF-kappaB in Waldenstrom macroglobulinemia. Blood. 2008;111:5068–5077. doi: 10.1182/blood-2007-09-115170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treon SP, Hunter ZR, Matous J, et al. Multicenter clinical trial of bortezomib in relapsed/refractory Waldenstrom's macroglobulinemia: Results of WMCTG Trial 03-248. Clin Cancer Res. 2007;13:3320–3325. doi: 10.1158/1078-0432.CCR-06-2511. [DOI] [PubMed] [Google Scholar]
- 9.Chen CI, Kouroukis CT, White D, et al. Bortezomib is active in patients with untreated or relapsed Waldenstrom's macroglobulinemia: A phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1570–1575. doi: 10.1200/JCO.2006.07.8659. [DOI] [PubMed] [Google Scholar]
- 10.Dimopoulos MA, Anagnostopoulos A, Kyrtsonis MC, et al. Bortezomib in Waldenstrom's macroglobulinemia. Haematologica. 2005;90:1655–1658. [PubMed] [Google Scholar]
- 11.Goy A, Younes A, McLaughlin P, et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:667–675. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 12.Byrd JC, White CA, Link B, et al. Rituximab therapy in Waldenstrom's macroglobulinemia: Preliminary evidence of clinical activity. Ann Oncol. 1999;10:1525–1527. doi: 10.1023/a:1008350208019. [DOI] [PubMed] [Google Scholar]
- 13.Foran JM, Rohatiner AZ, Cunningham D, et al. European phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. J Clin Oncol. 2000;18:317–324. doi: 10.1200/JCO.2000.18.2.317. [DOI] [PubMed] [Google Scholar]
- 14.Treon SP, Agus DB, Link B, et al. CD20-directed antibody-mediated immunotherapy induces responses and facilitates hematologic recovery in patients with Waldenstrom's macroglobulinemia. J Immunother. 2001;24:272–279. [PubMed] [Google Scholar]
- 15.Gertz MA, Rue M, Blood E, et al. Multicenter phase 2 trial of rituximab for Waldenstrom macroglobulinemia (WM): An Eastern Cooperative Oncology Group Study (E3A98) Leuk Lymphoma. 2004;45:2047–2055. doi: 10.1080/10428190410001714043. [DOI] [PubMed] [Google Scholar]
- 16.Dimopoulos MA, Zervas C, Zomas A, et al. Treatment of Waldenstrom's macroglobulinemia with rituximab. J Clin Oncol. 2002;20:2327–2333. doi: 10.1200/JCO.2002.09.039. [DOI] [PubMed] [Google Scholar]
- 17.Treon SP, Emmanouilides C, Kimby E, et al. Extended rituximab therapy in Waldenström's macroglobulinemia. Ann Oncol. 2005;16:132–138. doi: 10.1093/annonc/mdi022. [DOI] [PubMed] [Google Scholar]
- 18.Attal M, Harousseau JL, Facon T, et al. Single versus double transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 19.Durie BGM, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 20.Niesvizky R, Richardson PG, Rajkumar V, et al. The relationship between quality of response and clinical benefit for patients treated on the bortezomib arm of the international, randomized, phase 3 APEX trial in relapsed multiple myeloma. Br J Haematol. 2008;143:46–53. doi: 10.1111/j.1365-2141.2008.07303.x. [DOI] [PubMed] [Google Scholar]
- 21.Anderson KC, Kyle RA, Rajkumar SV, et al. Clinically relevant endpoints and new drug approvals for multiple myeloma. Leukemia. 2008;22:231–239. doi: 10.1038/sj.leu.2405016. [DOI] [PubMed] [Google Scholar]
- 22.Treon SP, Branagan AR, Ioakimidis L, et al. Long term outcomes to fludarabine and rituximab in Waldenstrom's macroglobulinemia. Blood. 2009;113:3673–3678. doi: 10.1182/blood-2008-09-177329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Treon SP, Gertz MA, Dimopoulos M, et al. Update on treatment recommendations from the Third International Workshop on Waldenstrom's macroglobulinemia. Blood. 2006;107:3442–3446. doi: 10.1182/blood-2005-02-0833. [DOI] [PubMed] [Google Scholar]
- 24.Dimopoulos MA, Anagnostopoulos A, Kyrtsonis MC, et al. Primary treatment of Waldenstrom macroglobulinemia with dexamethasone, rituximab, and cyclophosphamide. J Clin Oncol. 2007;25:3344–3349. doi: 10.1200/JCO.2007.10.9926. [DOI] [PubMed] [Google Scholar]
- 25.Treon SP, Soumerai JD, Branagan AR, et al. Thalidomide and rituximab in Waldenstrom's macroglobulinemia. Blood. 2008;112:4452–4457. doi: 10.1182/blood-2008-04-150854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimopoulos MA, Gertz M, Kastritis E, et al. Update on treatment recommendations from the Fourth International Workshop on Waldenstrom's Macroglobulinemia. J Clin Oncol. 2009;27:120–126. doi: 10.1200/JCO.2008.17.7865. [DOI] [PubMed] [Google Scholar]
- 27.Leleu X, Soumerai J, Roccaro A, et al. Increased incidence of transformation and myelodysplasia/acute leukemia in patients with Waldenström macroglobulinemia treated with nucleoside analogs. J Clin Oncol. 2009;27:250–255. doi: 10.1200/JCO.2007.15.1530. [DOI] [PubMed] [Google Scholar]
- 28.LeBlond V, Tamburini J, Levy V, et al. Incidence of disease transformation and development of MDS/AML in 165 patients with Waldenstrom macroglobulinemia treated with fludarabine based regimen in three studies. Blood. 2007;110:388a. abstr 1291. [Google Scholar]
- 29.Rose AL, Smith BE, Maloney DG. Glucocorticoids and rituximab in vitro: Synergistic direct antiproliferative and apoptotic effects. Blood. 2002;100:1765–1773. [PubMed] [Google Scholar]
- 30.Wang M, Han XH, Zhang L, et al. Bortezomib is synergistic with rituximab and cyclophosphamide in inducing apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leukemia. 2008;22:179–185. doi: 10.1038/sj.leu.2404959. [DOI] [PubMed] [Google Scholar]
- 31.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 32.Kyle RA, Treon SP, Alexanian R, et al. Prognostic markers and criteria to initiate therapy in Waldenström's macroglobulinemia: Consensus Panel Recommendations from the Second International Workshop on Waldenström's Macroglobulinemia. Semin Oncol. 2003;30:116–120. doi: 10.1053/sonc.2003.50038. [DOI] [PubMed] [Google Scholar]
- 33.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 34.Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–3120. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 35.Treon SP, Branagan AR, Hunter Z, et al. Paradoxical increases in serum IgM and viscosity levels following rituximab in Waldenstrom's macroglobulinemia. Ann Oncol. 2004;15:1481–1483. doi: 10.1093/annonc/mdh403. [DOI] [PubMed] [Google Scholar]
- 36.Ghobrial IM, Fonseca R, Greipp PR, et al. Initial immunoglobulin M “flare” after rituximab therapy in patients with Waldenstrom macroglobulinemia: An Eastern Cooperative Oncology Group Study. Cancer. 2004;101:2593–2598. doi: 10.1002/cncr.20658. [DOI] [PubMed] [Google Scholar]
- 37.Kimby E, Treon SP, Anagnostopoulos A, et al. Update on recommendations for assessing response from the Third International Workshop on Waldenstrom's Macroglobulinemia. Clin Lymphoma Myeloma. 2006;6:380–383. doi: 10.3816/CLM.2006.n.013. [DOI] [PubMed] [Google Scholar]
- 38.Ioakimidis L, Patterson CJ, Soumerai JD, et al. Comparative outcomes to CHOP-R, CVP-R, CP-R in patients with Waldenstrom's macroglobulinemia. Clin Lymphoma Myeloma. 2009;9:62–66. doi: 10.3816/CLM.2009.n.016. [DOI] [PubMed] [Google Scholar]
- 39.Buske C, Hoster E, Dreyling M, et al. The addition of rituximab to front-line therapy with CHOP (R-CHOP) results in a higher response rate and longer time to treatment failure in patients with lymphoplasmacytic lymphoma: Results of a randomized trial of the German Low-Grade Lymphoma Study Group (GLSG) Leukemia. 2009;23:153–161. doi: 10.1038/leu.2008.261. [DOI] [PubMed] [Google Scholar]
- 40.Nichols GL, Savage DG. Timing of rituximab/fludarabine in Waldenstrom's macroglobulinemia may avert hyperviscosity. Blood. 2004;104:237b. abstr 4612. [Google Scholar]
- 41.Treon SP, Soumerai JD, Branagan AR, et al. Lenalidomide and rituximab in Waldenstrom's macroglobulinemia. Clin Cancer Res. 2009;15:355–360. doi: 10.1158/1078-0432.CCR-08-0862. [DOI] [PubMed] [Google Scholar]
- 42.Strauss SJ, Higginbottom K, Juliger S, et al. Bortezomib therapy in patients with relapsed or refractory lymphoma: Potential correlation of in vitro sensitivity and tumor necrosis alpha with clinical activity. J Clin Oncol. 2006;24:2105–2112. doi: 10.1200/JCO.2005.04.6789. [DOI] [PubMed] [Google Scholar]
- 43.O'Connor OA, Wright J, Moscowitz C, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent lymphoma or mantle cell lymphoma. J Clin Oncol. 2005;23:676–684. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 44.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 45.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high dose dexamethasone for multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 46.Ghobrial IM, Matous J, Padmanabhan S, et al. Phase II trial of combination bortezomib and rituximab in relapsed and/or refractory Waldenstrom macroglobulinemia. Blood. 2008;112:302. abstr 832. [Google Scholar]
- 47.Chanan-Khan A, Sonneveld P, Schuster MW, et al. Analysis of herpes zoster events among bortezomib treated patients in the phase III APEX study. J Clin Oncol. 2008;26:4784–4790. doi: 10.1200/JCO.2007.14.9641. [DOI] [PubMed] [Google Scholar]