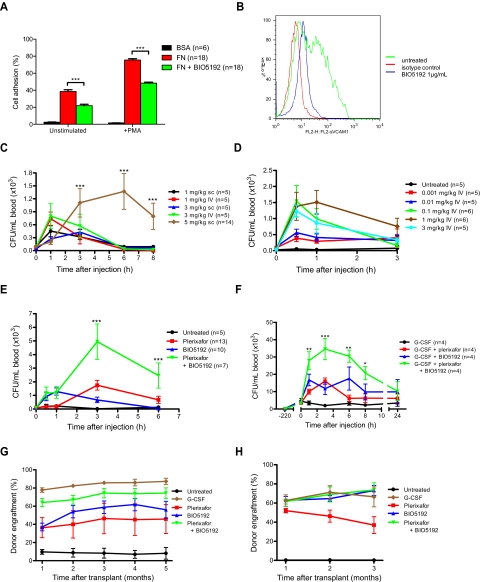
Figure 1.
Mobilization of hematopoietic stem and progenitor cells by BIO5192. (A) Calcein-AM labeled A20 cells were seeded in bovine serum albumin (BSA)–coated or fibronectin-coated plates and treated with phorbol 12-myristate 13-acetate and/or BIO5192 1 μg/mL. Cell adhesion is expressed as the percentage of fluorescence after removal of unbound cells compared with fluorescence of the total applied cells. (B) A20 cells incubated with recombinant human VCAM-1/Fc chimera protein plus or minus BIO5192 1 μg/mL for 30 minutes. Binding of VCAM-1 was detected using phycoerythrin-donkey anti–human Fc analyzed by fluorescence-activated cell sorter and compared with a phycoerythrin-conjugated donkey IgG (isotype control). (C-E) Colony-forming cell assays. C57BL/6J x 129Sv/J F1 mice were analyzed for peripheral blood CFU-GM after treatment with (C) plerixafor at 1, 3, or 5 mg/kg subcutaneously or intravenously. (D) BIO5192 at 0.001, 0.01, 0.1, 1, or 3 mg/kg intravenously. (E) Plerixafor 5 mg/kg subcutaneously and BIO5192 1 mg/kg intravenously alone or in combination or (F) G-CSF 250 μg/kg per day × 5 days alone, in combination with plerixafor 5 mg/kg subcutaneously, or BIO5192 1 mg/kg intravenously or the 3-drug combination. (G) Competitive repopulation assay. Lethally irradiated CD45.1+/CD45.2+ mice received transplants of 0.5 × 106 congenic CD45.1+ bone marrow competitor cells plus PBMCs from untreated mice or those mobilized with 250 μg/kg per day of G-CSF × 5 days, BIO5192 mg/kg intravenously, plerixafor 5 mg/kg subcutaneously, or the combination of BIO5192 1 mg/kg intravenously and plerixafor 5 mg/kg subcutaneously (n = 3 mice/group). Peripheral blood was harvested on day 5 for G-CSF–treated mice, 1 hour after injection for BIO5192, and 3 hours after injection for plerixafor and plerixafor + BIO5192–treated mice. The contribution of mobilized cell populations to hematopoiesis was determined by flow cytometry for CD45.2+ donor cells. (H) Secondary transplantation. A total of 106 bone marrow cells from primary transplant recipients were injected into lethally irradiated C57BL/6 secondary mice (CD45.1+/CD45.2+, n = 3 mice/group). Engraftment was assessed monthly for 3 months by peripheral blood flow cytometry for CD45.2+ donor cells. Data are mean ± SEM. *P < .05; **P < .01; ***P < .001.