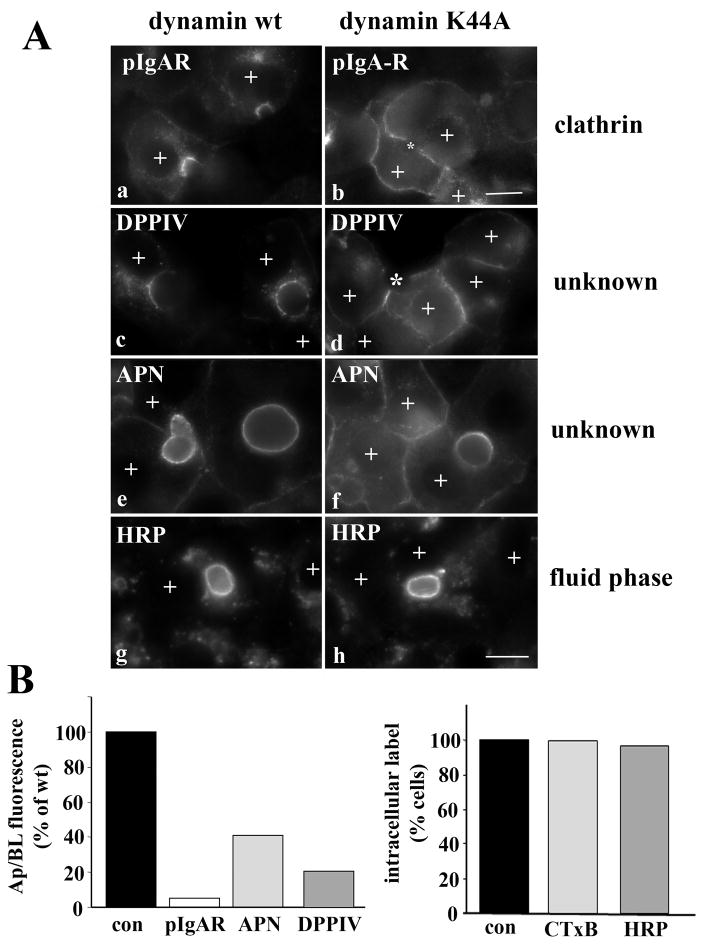
Figure 7.
Internalization of the single spanning apical residents is clathrin-dependent. A, WIF-B cells were co-infected for 30 min at 37°C with recombinant adenovirus particles encoding the tetracycline repressible transactivator and wildtype (a, c, e and g) or K44A dominant negative dynamins (b, d, f, and h) under the control of the tetracycline responsive element. Cells were additionally infected with pIgA-R (a and b) or DPPIV (c and d) viruses. After washing, the cells were incubated an additional 18–24 h to allow dynamin expression. Live cells were labeled with anti-pIgA-R (a and b), anti-DPP IV (c and d) or anti-APN (e and f) antibodies and the antigen-antibody complexes chased for 90 min. Cells were also continuously labeled for 2 h with 5 mg/ml HRP in serum free medium (g and h). Cells expressing the recombinant dynamin adenoviruses are indicated with a “+” symbol. Asterisks in panels b and d are marking unlabeled BCs in cells overexpressing dynamin K44A. Bar = 10 μm B, Cells were infected with wild type of K44A dynamin viruses as described in A, and internalization of the indicated markers was monitored by epifluorescence. Apical to basolateral fluorescence intensities were measured for pIgA-R, DPPIV and APN in dynamin expressing cells as described in Figure 1A. For CTxB and HRP, dynamin-expressing cells were scored for the presence of labeled intracellular puncta, and percent positive cells calculated.