Fig. 2.
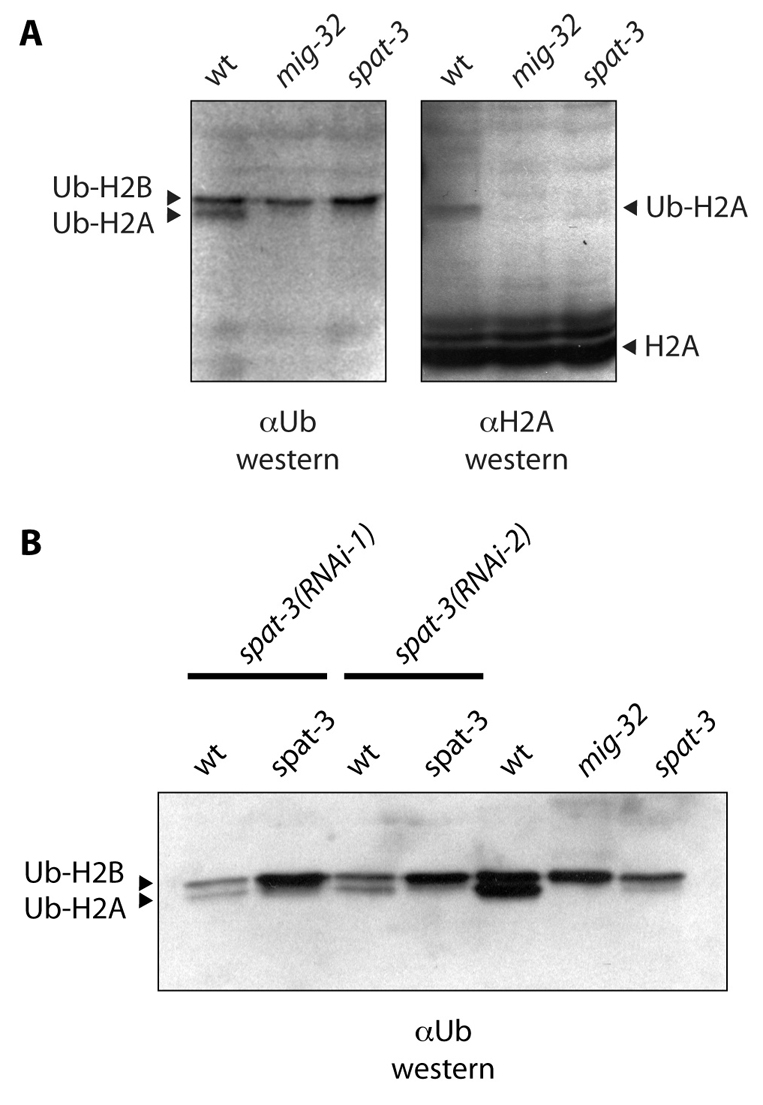
mig-32 is required for ubiquitylation of histone H2A. (A) Western blot analysis of acid-extracted histones from wild-type, mig-32(n4275) and spat-3(gk22) mutant C. elegans. The filter shown in the left panel was probed with anti-ubiquitin antibody. Arrowheads indicate ubiquitylated H2A and H2B. In the right panel, this blot was stripped and probed with anti-H2A antibody. Arrowheads indicate H2A and ubiquitylated H2A. (B) spat-3 mutants have greatly reduced levels of ubiquitylated H2A. A western blot using anti-ubiquitin antibody detects a trace amount of ubiquitylated H2A in most preparations of acid-extracted histones from spat-3(gk22) mutants, consistent with this allele being strong loss-of-function, but not null. A spat-3(RNAi) construct was fed to wild-type animals in two experiments [spat-3(RNAi-1) and spat-3(RNAi-2)]. The RNAi construct reduces, but does not eliminate, ubiquitylated H2A levels. Loading was not equal in all lanes of this blot.
