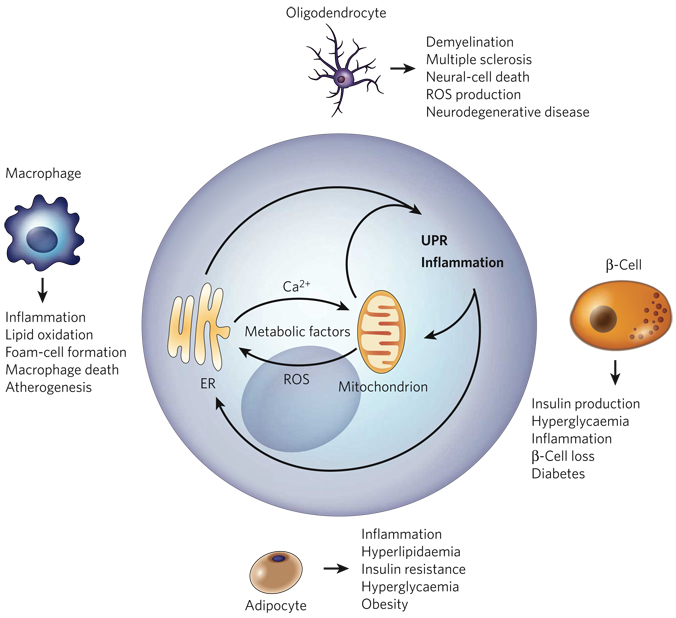
Figure 6. The ‘ER-stress–inflammation’ loop in specialized cells.
In specialized cells that secrete large amounts of protein — such as macrophages, adipocytes, β-cells and oligodendrocytes — the UPR and inflammatory-response signalling can be triggered by a chronic excess of extracellular and/or intracellular metabolic factors, such as lipids, glucose, cytokines, hormones, non-esterified fatty acids and neurotransmitters. More specifically, such metabolic factors stimulate protein synthesis, calcium signalling and ROS production by targeting the mitochondria and the ER in these cells (Fig. 5). The increased protein-folding demand and the signalling involving calcium and ROS induce the UPR and inflammatory-response signalling, leading to the transcription of genes whose products mount a broader inflammatory response. An excess of metabolic factors can further boost the UPR and inflammation, contributing to impaired lipid and glucose metabolism, insulin resistance and apoptosis. This forward ER-stress– inflammation loop could also further promote inflammatory stress signalling and contribute to the metabolic deterioration that is associated with atherosclerosis, obesity, type 2 diabetes and neurodegenerative diseases, depending on the cell type involved.