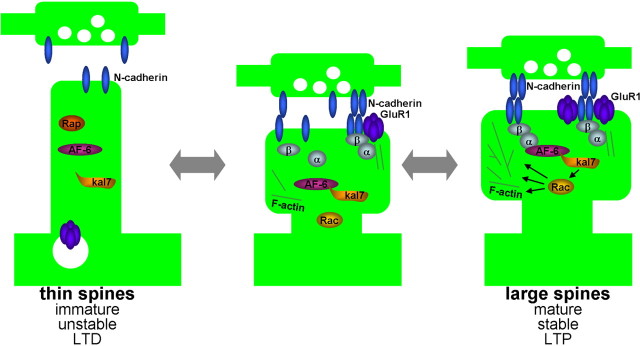
Figure 9.
Model of the role of N-cadherin and associated proteins, AF-6, and kalirin-7 in the dynamic coordination of synaptic adhesion and spine morphology. Clustering of N-cadherin at synapses during synapse maturation, stabilization, or potentiation recruits AF-6, which in turn recruits the Rac1-GEF kalirin-7. Kalirin-7 then activates Rac1 locally, to induce spine enlargement. These large spines are also rich in AMPA receptors. Conversely, weakening of N-cadherin adhesion during synapse destabilization, turnover, experience-dependent plasticity, or synaptic depression, results in the release of AF-6 and kalirin-7 from adhesion sites, leading to reduced local Rac1 activation, resulting in thinner and longer spines, with reduced AMPA receptor content.