Abstract
OBJECTIVES:
Endovascular aortic aneurysm repair (EVAR) is a current valid treatment option for patients with abdominal aortic aneurysms (AAAs). The success of EVAR depends on the selection of appropriate patients, which requires detailed knowledge of the patient’s vascular anatomy and preoperative planning. Three-dimensional (3D) models of AAA using a rapid prototyping technique were developed to help surgical trainees learn how to plan for EVAR more effectively.
METHOD:
Four cases of AAA were used as prototypes for the models. Nine questions associated with preoperative planning for EVAR were developed by a group of experts in the field of endovascular surgery. Forty-three postgraduate trainees in general surgery participated in the present study. The participants were randomly assigned into two groups. The ‘intervention’ group was provided with the rapid prototyping AAA models along with 3D computed tomography (CT) corresponding to the cases of the test, while the control group was provided with 3D CTs only.
RESULTS:
Differences in the scores between the groups were tested using the unpaired t test. The mean test scores were consistently and significantly higher in the 3D CT group with models compared with the 3D CT group without models for all four cases. Age, year of training, sex and previous EVAR experience had no effect on the scores.
CONCLUSION:
The 3D aortic aneurysm model constructed using the rapid prototype technique may significantly improve the ability of trainees to properly plan for EVAR.
Keywords: CT angiogram, Preoperative planning, Visual-spatial ability
The development of modern angiographic methods, such as computed tomography (CT) angiography and magnetic resonance angiography, has had a major effect on the practice of vascular surgery. Endovascular aortic aneurysm repair (EVAR) is an emerging treatment for patients with abdominal aortic aneurysms (AAAs) (1,2). EVAR is the luminal exclusion of an aneurysm from circulatory flow using a conduit (endograft) inserted from a remote access vessel and deployed under fluoroscopic guidance (3–5). Over the past two decades, vascular surgeons have embraced this technology and adopted this approach for the treatment of aneurysms (6,7).
Preoperative planning is a crucial part of endovascular aneurysm repair (8,9). Success of EVAR depends on the selection of appropriate patients, which requires detailed knowledge of the patient’s vascular anatomy such that an appropriately sized graft is chosen (10). Errors in preoperative planning can lead to attachment site endoleak, graft migration or other complications, which may result in conversion to open surgery or other secondary interventions, and even aneurysm rupture (11,12). It is ironic that a less invasive method of aneurysm repair is associated with more invasive preoperative preparation.
Three-dimensional (3D) CT with reformatted images perpendicular to the blood flow is the method of choice for aortic aneurysm assessment and image-based planning before EVAR (13). However, there can be considerable interobserver variability with this imaging method (10). Successful preoperative evaluation of patients for EVAR also requires considerable training time and the ability to visualize 3D structures from two-dimensional representations (14). We developed 3D models of AAAs using a rapid prototyping technique to enable surgical trainees to plan for EVAR more effectively. To our knowledge, the present study is the first application of this technology in an educational context.
METHODS
Rapid prototype aortic aneurysm models
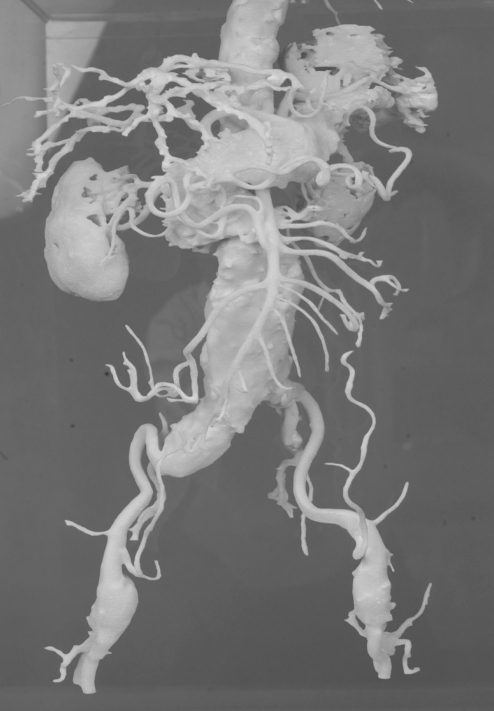
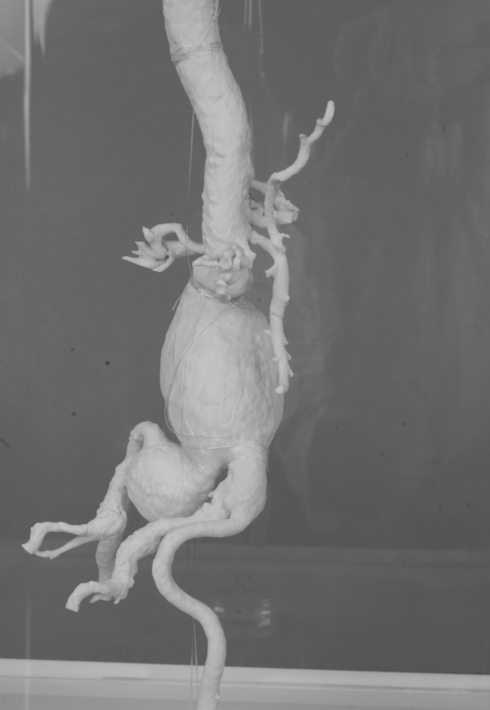
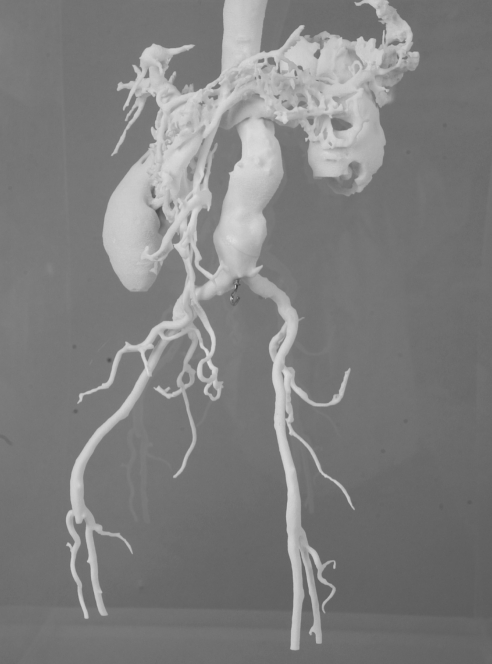
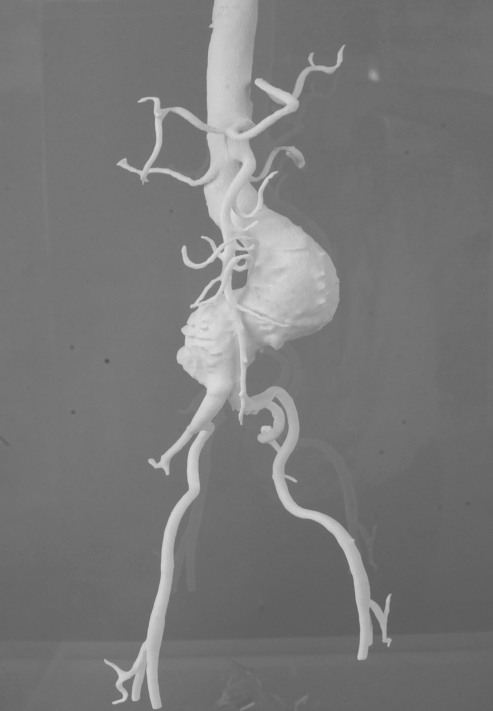
Four cases of infrarenal aortic aneurysm were used as prototypes for the models. The aortic aneurysm models were manufactured life-size, with the original configuration based on the information obtained from 3D CTs by a rapid prototyping technique (Figures 1–4).
Figure 1).
Abdominal aortic aneurysm model 1
Figure 4).
Abdominal aortic aneurysm model 4
Rapid prototyping is an advanced production process that can fabricate physical objects directly from 3D computer-aided design models. This technology enables the fabrication of anatomical models within a short time without tooling (tool-less manufacturing). During the process, layers of materials are added (additive technology) until the whole model is finished in a single step.
The 3D CTs used for the fabrication of 3D physical vascular models were obtained with a slice thickness of 2 mm or less, to ensure high accuracy and precision. The CT data were transferred into medical image processing software (eg, Mimics; Materialise NV, Belgium) to construct a 3D computer-aided design model. The segmentation technique was used to determine the boundary of hard and soft tissues in the region of interest. The physical model was then fabricated via the rapid prototyping technique within 2 h to 6 h, depending on the size of the model.
Testing for the ability to plan for EVAR
Nine questions associated with preoperative planning for EVAR were developed by a group of experts in the field of endovascular surgery (Table 1). These questions were considered to have sufficient face validity to assess the ability of each trainee in planning for EVAR. Four clinical scenarios based on real patients with AAA were presented to the trainees. The nine questions for each scenario had only yes or no answers. The correct answers were agreed on by the same group of experts who developed the questions. The possible scores of the test ranged from 0 (all incorrect) to 9 (all correct).
Table 1.
Questions used for testing the ability to plan for endovascular aortic aneurysm repair (EVAR)
| Questions |
|---|
|
Participants
Forty-three postgraduate trainees in general surgery participated in the present study. There were 12 first-year, eight second-year, eight third-year, eight fourth-year and seven fifth-year surgical residents who were relative novices in vascular surgery. Some of the trainees had assisted in the performance of EVAR before the study. These trainees were not excluded, but the EVAR experience was taken into account in the analysis of the test results. The participants were randomly assigned into two groups, using randomized blocks of three and four participants. The ‘intervention’ group was provided with the rapid prototyping aortic aneurysm models along with 3D CTs corresponding to the scenarios of the test to use in the preoperative planning, while the control group was provided with 3D CTs only. Participants were requested to answer all questions.
Statistical analysis
Test scores and age were summarized as mean ± SD. Year of residency training, previous operative experience with EVAR placement as a first assistant and sex were summarized as counts and percentages. Differences in the scores between the group provided with both 3D CTs and an aneurysm model (M+3D CT) and the group provided with 3D CTs only were tested using the unpaired t test. The mean differences in the scores between the two groups, after adjusting for baseline characteristics as well as the problem scenarios, were estimated using mixed model linear regression with a Gaussian random effect at the resident level. All statistical analyses were performed using Stata version 9 (Stata Corp, USA). Statistical significance was defined as a two-tailed P≤0.05.
RESULTS
Characteristics of participants are presented in Table 2. There were no significant differences between the two randomly assigned groups in terms of these characteristics. The mean age of the participants was 29.2±2.7 years in the M+3D CT group and 29.2±2.5 years in the 3D CT only group. In the M+3D CT group, 32% of the participants had previously assisted in EVAR cases, while 43% in the 3D CT only group had the same experience.
Table 2.
Baseline characteristics of trainees
| Baseline characteristic | 3D CT + model, n=22 | 3D CT only, n=21 |
|---|---|---|
| Age, years | 29.2±2.7 | 29.2±2.5 |
| Year of residency training | ||
| 1 | 6 (27) | 6 (29) |
| 2 | 4 (18) | 4 (19) |
| 3 | 4 (18) | 4 (19) |
| 4 | 4 (18) | 4 (19) |
| 5 | 4 (18) | 3 (14) |
| Sex, male/female | 20/2 (91/9) | 18/3 (86/14) |
| EVAR experience, yes | 7 (32) | 9 (43) |
Age presented as mean ± SD; all other values presented as n (%). 3D Three-dimensional; CT Computed tomography; EVAR Endovascular aortic aneurysm repair
The mean test scores were consistently and significantly higher in the M+3D CT group than in the 3D CT group for all four scenarios (Table 3). The mean scores in the M+3D CT group were 7.3±0.78, 8.7±0.48, 7.5±0.91 and 7.5±0.91 for scenarios 1, 2, 3 and 4, respectively. The mean scores in the 3D CT only group were 5.5±1.2, 5.7±1.0, 5.1±1.3 and 5.4±0.98 for scenarios 1, 2, 3 and 4, respectively.
Table 3.
Mean scores for each test question
| Test scenario | 3D CT + model, n=22 | 3D CT only, n=21 | Mean difference (95% CI) | P* |
|---|---|---|---|---|
| 1 | 7.3±0.78 | 5.5±1.2 | 1.8 (1.2 to 2.4) | <0.001 |
| 2 | 8.7±0.48 | 5.7±1.0 | 3.0 (2.5 to 3.4) | <0.001 |
| 3 | 7.5±0.91 | 5.1±1.3 | 2.4 (1.7 to 3.1) | <0.001 |
| 4 | 7.5±0.91 | 5.4±0.98 | 2.1 (1.4 to 2.6) | <0.001 |
Data presented as mean ± SD.
P by unpaired t test. 3D Three-dimensional; CT Computed tomography
Table 4 presents the mean difference in the scores across all scenarios after adjusting for baseline characteristics, based on the statistical model previously described. On average, trainees in the M+3D CT group were able to answer 2.3 more questions correctly than those in the 3D CT only group. Age, year of training, sex and previous EVAR experience had no effect on the scores. Scenario 2 (Table 4) seemed to be easier to answer than the other scenarios, especially for the trainees in the M+3D CT group.
Table 4.
Mean difference in test scores between groups after adjusting for baseline factors and scenario questions
| Group and baseline factors | Mean value (95% CI) | P |
|---|---|---|
| Baseline score | 5.1 (2.7 to 7.5) | <0.001 |
| Group difference | 2.3 (2.0 to 2.6) | <0.001 |
| Age (per higher year) | 0.01 (−0.08 to 0.11) | 0.799 |
| Training difference (per higher year) | 0.07 (−0.10 to 0.25) | 0.421 |
| Sex difference (male versus female) | −0.39 (−0.89 to 0.12) | 0.135 |
| EVAR versus no experience | −0.12 (−0.48 to 0.25) | 0.525 |
| Scenario 2 versus scenario 1 | 0.79 (0.38 to 1.2) | <0.001 |
| Scenario 3 versus scenario 1 | −0.16 (−0.57 to 0.25) | 0.434 |
| Scenario 4 versus scenario 1 | 0.02 (−0.38 to 0.43) | 0.911 |
EVAR Endovascular aortic aneurysm repair
DISCUSSION
Accurate preoperative planning for EVAR is extremely important to ensure proper selection of patients, stent-graft type and potential intraoperative adjuncts. The ability to accurately and consistently select appropriate patients is critical for the success of EVAR (7,9,10). The present study demonstrated that the 3D aortic aneurysm model constructed using the rapid prototype technique can significantly improve the ability of trainees to properly plan for EVAR.
Visualization of the 3D structure of AAAs may be difficult for trainees who are novices to aortic aneurysm surgery. It is difficult to measure how a physician perceives two-dimensional radiological images, which may also be influenced by the level of training or experience. The results of the present study may be indirect evidence that the visual-spatial ability of surgical trainees may not be adequate for accurately interpreting 3D CT angiograms across all years of residency training. Adding the 3D aneurysm models to the preoperative radiological images could help improve the ability of trainees to visualize the diseased aorta, and was clearly useful in the planning for EVAR. One important question that the present study could not answer is whether learning with such models can improve future planning by the trainees when the models are no longer available.
It is unknown whether a similar 3D model of the aortic aneurysm can also help the expert vascular surgeon to better plan for EVAR. A study could be performed in which experts are asked to rate their confidence in managing aortic aneurysm cases with various anatomical complexities and complications when provided with the aneurysm models based on the imaging information. This could be compared with a control group of experts not provided with aneurysm models.
Because of the cost and practicality issues, the aneurysm models can only be used for educational purposes. It is not currently possible or practical to produce aneurysm models routinely for clinical use. A collection of models and corresponding radiological images of interesting or complex case studies can be assembled to help trainees gain valuable learning experience. The models can also be used in scientific presentations to make the case examples more concrete and real to the audience.
CONCLUSION
The AAA model is likely to be useful for novices and trainees to help visualize the abnormal vascular anatomy more accurately. The model can help trainees improve their ability to plan for EVAR.
Figure 2).
Abdominal aortic aneurysm model 2
Figure 3).
Abdominal aortic aneurysm model 3
Acknowledgments
The authors thank Professor Amnuay Thihapandha for helping with the English editing of this manuscript.
REFERENCES
- 1.Danjoux NM, Martin DK, Lehoux PN, et al. Adoption of an innovation to repair aortic aneurysms at a Canadian hospital: A qualitative case study and evaluation. BMC Health Serv Res. 2007;15:7–182. doi: 10.1186/1472-6963-7-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush RL, Mureebe L, Bohannon WT, Rutherford RB. The impact of recent European trials on abdominal aortic aneurysm repair: Is a paradigm shift warranted? J Surg Res. 2008;148:264–71. doi: 10.1016/j.jss.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Kubin K, Sodeck GH, Teufelsbauer H, et al. Endovascular therapy of ruptured abdominal aortic aneurysm: Mid- and long-term results. Cardiovasc Intervent Radiol. 2008;31:496–503. doi: 10.1007/s00270-007-9253-9. [DOI] [PubMed] [Google Scholar]
- 4.Mastracci TM, Garrido-Olivares L, Cinà CS, Clase CM. Endovascular repair of ruptured abdominal aortic aneurysms: A systematic review and meta-analysis. J Vasc Surg. 2008;47:214–21. doi: 10.1016/j.jvs.2007.07.052. [DOI] [PubMed] [Google Scholar]
- 5.Wyers MC, Fillinger MF, Schermerhorn ML, et al. Endovascular repair of abdominal aortic aneurysm without preoperative arteriography. J Vasc Surg. 2003;38:730–8. doi: 10.1016/s0741-5214(03)00552-4. [DOI] [PubMed] [Google Scholar]
- 6.De Rango P, Cao P, Parlani G, Verzini F, Brambilla D. Outcome after endografting in small and large abdominal aortic aneurysms: A meta-analysis. Eur J Vasc Endovasc Surg. 2008;35:162–72. doi: 10.1016/j.ejvs.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Murphy EH, Arko FR. Technical tips for abdominal aortic endografting. Semin Vasc Surg. 2008;21:25–30. doi: 10.1053/j.semvascsurg.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Boult M, Maddern G, Barnes M, Fitridge R. Factors affecting survival after endovascular aneurysm repair: Results from a population based audit. Eur J Vasc Endovasc Surg. 2007;34:156–62. doi: 10.1016/j.ejvs.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Parker MV, O’Donnell SD, Chang AS, et al. What imaging studies are necessary for abdominal aortic endograft sizing? A prospective blinded study using conventional computed tomography, aortography, and three-dimensional computed tomography. J Vasc Surg. 2005;41:199–205. doi: 10.1016/j.jvs.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Hoornweg LL, Wisselink W, Vahl AC, et al. Interobserver and intraobserver variability of interpretation of CT-angiography in patients with a suspected abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg. 2008;35:295–300. doi: 10.1016/j.ejvs.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Frego M, Lumachi F, Bianchera G, et al. Risk factors of endoleak following endovascular repair of abdominal aortic aneurysm. A multicentric retrospective study. In Vivo. 2007;21:1099–102. [PubMed] [Google Scholar]
- 12.Szmidt J, Galazka Z, Rowinski O, et al. Late aneurysm rupture after endovascular abdominal aneurysm repair. Interact Cardiovasc Thorac Surg. 2007;6:490–4. doi: 10.1510/icvts.2007.152447. [DOI] [PubMed] [Google Scholar]
- 13.Sprouse LR, II, Meier GH, III, Parent FN, et al. Is three-dimensional computed tomography reconstruction justified before endovascular aortic aneurysm repair? J Vasc Surg. 2004;40:443–7. doi: 10.1016/j.jvs.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Sidhu RS, Tompa D, Jang R, et al. Interpretation of three-dimensional structure from two-dimensional endovascular images: Implications for educators in vascular surgery. J Vasc Surg. 2004;39:1305–11. doi: 10.1016/j.jvs.2004.02.024. [DOI] [PubMed] [Google Scholar]