Abstract
Epichlorohydrin (ECH), an important industrial chemical, is a bifunctional alkylating agent with the potential to form DNA cross-links. Occupational exposure to this suspect carcinogen leads to chromosomal aberrations, and ECH has been shown previously to undergo reaction with DNA in vivo and in vitro.We used denaturing polyacrylamide gel electrophoresis to monitor the possible formation of interstrand cross-links within DNA oligomers by ECH and the related compound, epibromohydrin (EBH). Although both compounds did indeed form cross-links between deoxyguanosine residues, EBH was a more efficient cross-linker than ECH. The optimal pH for cross-linking also varied, with ECH more efficient at pH 5.0 and EBH more efficient at pH 7.0. Both agents were relatively flexible in the sequences targeted, with comparable efficiencies for 5′-GGC and 5′GC sites. Furthermore, interstrand cross-linking by the two optical isomers of ECH correlated with their relative cytotoxicities, with R-ECH about twice as potent as S-ECH.
Introduction
Bifunctional alkylating agents are among the most powerful antitumor drugs (1). Although these compounds form a variety of cellular lesions, DNA interstrand cross-links are believed to be the most cytotoxic, disrupting normal replication and transcription. This activity is beneficial when directed at cancer cells, yet patients who undergo treatment with cross-linking drugs have elevated risks of developing secondary cancers later in life (2, 3). Additionally, occupational exposure to bifunctional alkylating agents can elevate cancer risk for industrial workers. For example, the increased incidence of leukemia among workers in the synthetic rubber industry has been linked to DNA-reactive metabolites of 1,3-butadiene, such as diepoxybutane (DEB1; 1) (4-6). DEB cross-links distal deoxyguanosines at duplex 5′-GNC sequences, where N is any base (7, 8). This is the same sequence targeted by the antitumor drug mechlorethamine (HN2; 2) (9, 10), although the two agents differ in the influence of the flanking bases on cross-linking efficiency (11).
Epichlorohydrin (ECH; 3), another potential bifunctional alkylating agent, is widely used in the production of epoxy resins, glycerine, elastomers, and specialty chemicals. In 2003, 903,000 metric tons of the compound was consumed (12), and it is classified as a probable human carcinogen (13). The genotoxicity of ECH has been demonstrated in industrial workers, who have increases in chromosomal aberrations, cell damage, and rates of certain cancers upon exposure (14-16). In cultured mammalian cells, ECH is mutagenic (17) and clastogenic (18) and induces neoplastic cell transformation (19). In test animals, ECH has been shown to cause papillomas and carcinomas (20) and to inhibit spermatogenesis (21). Understanding its mechanism of toxicity may clarify the risk of exposure for the estimated 250,000 industrial workers exposed to this compound annually in the United States alone (16).
Chart 1.
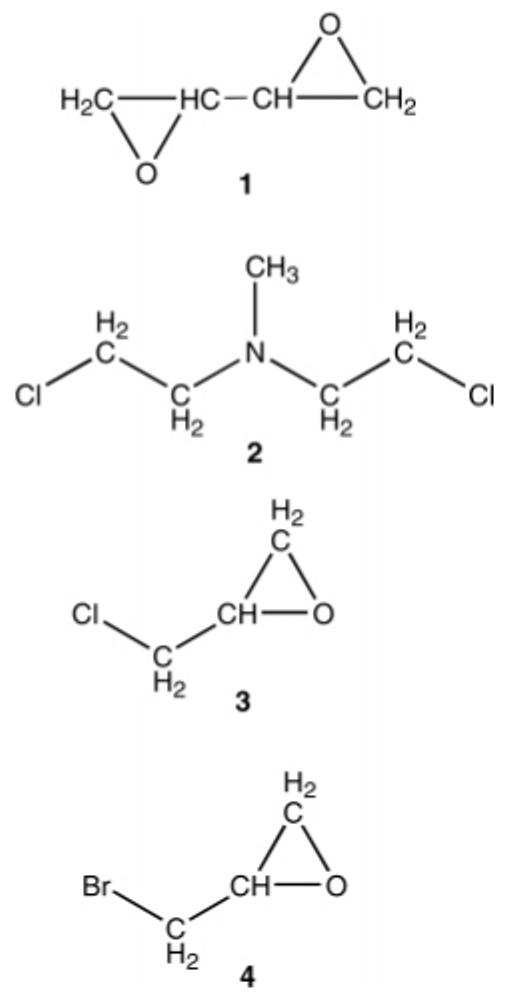
Cross-Linkers Relevant to This Study
The chloride and epoxide functionalities make ECH a “hybrid” of HN2 and DEB, although a three-atom chain has been proposed to be too short for interstrand cross-linking (22). However, there is evidence that ECH reacts with DNA, including binding covalently in vitro and in vivo (23-26) as well as inducing DNA strand breaks (27, 28). Studies with free bases have shown that ECH forms adducts prominently with N7 and O6 of guanine, and N6 and N3 of adenine (26, 29, 30-32). Furthermore, ECH has been shown to cross-link proteins (33).
Our goal in this study was to characterize possible DNA interstrand cross-linking by ECH, including any sequence preferences. We also examined the related compound epibromohydrin (EBH; 4) because its better leaving group makes it likely to be more reactive than ECH. Indeed, EBH has been reported to be more mutagenic than ECH in some test organisms (34). Our data support interstrand cross-linking at deoxyguanosine residues by each agent, with EBH more reactive than ECH. Both agents showed a decreased sequence preference relative to DEB and HN2. Differences in optimal pH for the two epihalohydrins suggest mechanistic differences in their reactions with DNA. Furthermore, the R-stereoisomer of ECH was about 2-fold more effective at cross-linking than the S-stereoisomer as well as being about twice as cytotoxic.
Experimental Procedures
Caution
ECH, EBH, and DEB are suspect carcinogens and must be handled appropriately.
Preparation of Radiolabeled DNA Duplexes
Oligonucleotides (Integrated DNA Technologies, Inc., Coralville, IA) were purified via 20% denaturing polyacrylamide gel electrophoresis (19:1 acrylamide/bisacrylamide; 40% urea) followed by the crush-and-soak procedure (35). 5′-End radiolabeling was achieved with [γ-32P]-ATP, T4 polynucleotide kinase, and 25 μg of one DNA strand under standard conditions (35), followed by ethanol precipitation. Twenty-five micrograms of the complementary strand was added to the radiolabeled pellet, and the appropriate amount of desired buffer was added to achieve a 100 μL total final volume after the addition of cross-linker. TE buffer (10 mM Tris-Cl and 1 mM EDTA), 0.1 M MES buffer, and 0.1 M sodium acetate buffer were used for reactions run at pH 7.0, 6.0, and 5.0, respectively. Samples were heated at 65 °C for 20 min, followed by benchtop cooling for 20 min to ensure annealing. 3′-End radiolabeling was achieved with [α-32P]-dATP, Klenow Exo Minus, and 50 μg of duplex DNA under standard conditions (35), followed by ethanol precipitation. Lyophilized samples were then dissolved in the appropriate buffer.
Cross-Linking Reactions
All cross-linkers were purchased from Sigma-Aldrich Chemical Co. (Milwaukee, WI). For 250 mM reactions, 2 μL of the desired cross-linking agent (ECH, EBH, or DEB) was added to each tube. After establishing optimal cross-linking conditions, we used sodium acetate (pH 5.0) for ECH reactions and TE buffer (pH 7.0) for EBH and DEB reactions. All reactions were run at 37 °C. For time trials, aliquots were removed and ethanol precipitated during the course of the reaction. When the optimal reaction time was established for a particular DNA and cross-linker, another reaction was performed for that time period and then stopped by ethanol precipitation. Optimal reaction times were as follows: ECH, 4-10 h; EBH, 4-6 h; and DEB, 45 min.
Separation of Cross-Links
Cross-linked oligonucleotides were run on 20% denaturing polyacrylamide gels (19:1 acrylamide/bisacrylamide, 50% urea) at 60 W and ambient temperature. Gels were dried for analysis via phosphorimagery (Amersham Biosciences STORM 840) or left wet for purification of alkylated DNA after autoradiography. Percent cross-linking was determined through volume analysis of the low-mobility bands in comparison to total DNA.
Piperidine Cleavage of Alkylated DNA
Gel-purified alkylated DNA was cleaved at sites of guanine N7 alkylation by heating at 90 °C in 10% aqueous piperidine for 30 min, followed by lyophilizing, dissolving in 40 μL of water, lyophilizing, dissolving in 25 μL of water, and lyophilizing again (35). Piperidine cleavage products were analyzed on 25% denaturing polyacrylamide gels (19:1 acrylamide/bisacrylamide, 50% urea) run at 60 W and 55 °C. After drying and phosphorimaging, the relative intensities of bands corresponding to cleavage at guanine residues were determined through volume analysis.
Cytotoxicity Assays
We used an adaptation of a standard protocol (36) to determine the cytotoxicity of R-ECH and S-ECH in 6C2 chicken erythro-progenitor cells. Briefly, confluent cells were plated at a 1:5 dilution with MEM Richter’s modification with l-glutamine and grown for 12-18 h (37 °C, 5% CO2) to ensure entry into the log phase of growth. R-ECH or S-ECH was added with mixing, and the cells were incubated for 4 h (37 °C, 5% CO2). Two hundred microliters aliquots were removed and mixed with 20 μL of 0.4% trypan blue in phosphate buffered saline (137 mM NaCl, 10 mM phosphate, and 2.7 mM KCl (pH 7.4)). Twenty microliter aliquots of dyed samples were loaded onto a hemocytometer, and the total number of cells and total number of viable cells per square were counted. The viable fraction was calculated for each square as the number of viable cells divided by the total number of cells. Results from all squares were averaged. At least four replicate counts were averaged for both R-ECH and S-ECH. The average viable fraction was plotted on a log scale versus ECH concentration, and the IC50 was calculated using the best-fit exponential curve. Flow cytometry was used to determine the distribution of cells in various stages of the cell cycle (G1, S, and G2/M) under the conditions used for the cytotoxicity assays (37).
Results
Determination of Reaction Conditions
We used denaturing polyacrylamide gel electrophoresis (dPAGE) to monitor possible ECH and EBH interstrand cross-linking within radiolabeled DNA oligomers. Conveniently, interstrand cross-links appear as low-mobility bands relative to the corresponding single-strands on denaturing gels (38). We began with Duplex A (Table 1), a 27 base-pair (bp) duplex containing the preferred core sequence for DEB cross-linking, 5′-GGCCC (11). For both ECH and EBH, denaturing gels revealed a low-mobility band growing in intensity over time that we attributed to an interstrand cross-linked product. Significant amounts of low-mobility product formed only after several hours, which is considerably longer than the 45 min required for DEB cross-linking of oligomers (11). Furthermore, ECH required longer incubation times than EBH for significant cross-linking to occur. Optimal reaction conditions for both ECH and EBH were a concentration of 250 mM and a temperature of 37 °C, similar to those for DEB. Higher concentrations of epihalohydrins led to increased production of high-mobility streaks of radioactivity on the gels, suggesting DNA degradation. Dose-dependent ECH-induced strand cleavage has been noted previously (28).
Table 1.
DNA Oligomers Used in These Studies
| Duplex A |
5′CGAAGGCCCAAGGCCCTAGGCCCATGC3′ 3′GCTTCCGGGTTCCGGGATCCGGGTACG5′ |
| Duplex B |
5′CGTTTAAGGCCCTTGGCCCTAGGCCCATGC3′ 3′TTCCGGGAACCGGGATCCGGGTACG |
| Duplex C |
5′AATATAAGCTTTAAAT3′ 3′TTATATTCGAAATTTA5′ |
| Duplex D |
5′AATATAGGCTTTAAAT3′ 3′TTATATCCGAAATTTA5′ |
| Duplex E |
5′AATATAGGGCTTAAAT3′ 3′TTATATCCCGAATTTA5′ |
| Duplex F |
5′TATATATTTATAGGCTATATTTATATT3′ 3′ATATATAAATATCCGATATAAATATAA5′ |
Interestingly, the pH dependence for the production of the putative cross-link varied for the two epihalohydrins, with ECH showing maximal cross-linking at pH 5.0 (Figure 1) and EBH showing maximal cross-linking at pH 7.0 (Figure 2). Moreover, the efficiency of EBH cross-linking was generally greater than that of ECH even at shorter reaction times under the optimal pH conditions for each agent. For example, after 8 h at pH 7.0, there was 4.2% low-mobility band for EBH, whereas after 12 h at pH 5.0, there was only 1.5% low-mobility band for ECH.
Figure 1.
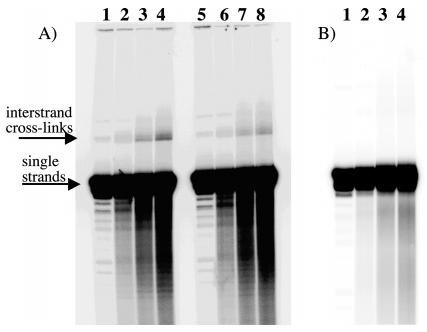
(Panel A) Reaction of ECH and Duplex A over time at pH 5.0 (lanes 1-4) and pH 6.0 (lanes 5-8) at 37 °C. Reaction aliquots were taken at time 0 (lanes 1 and 5), 4 h (lanes 2 and 6), 8 h (lanes 3 and 7), and 12 h (lanes 4 and 8). Presumed interstrand cross-links appear as low-mobility bands. (Panel B) Reaction of ECH and Duplex A over time at pH 7.0. Reaction aliquots were taken at time 0 (lane 1), 4 h (lane 2), 8 h (lane 3), and 12 h (lane 4).
Figure 2.
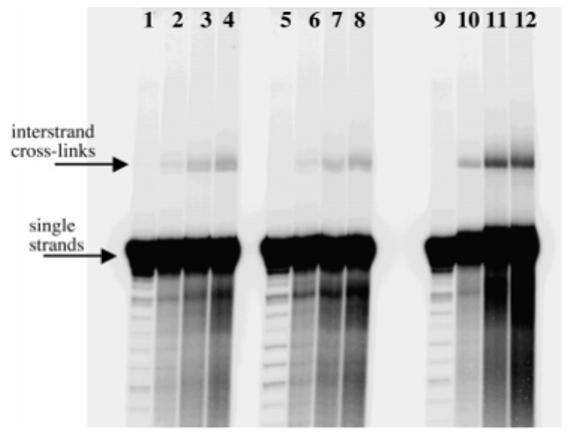
Reaction of EBH and Duplex A over time at pH 5.0 (lanes 1-4), pH 6.0 (lanes 5-8), and pH 7.0 (lanes 9-12) at 37 °C. Reaction aliquots were taken at time 0 (lanes 1, 5, and 9), 4 h (lanes 2, 6, and 10), 8 h (lanes 3, 7, and 11), and 12 h (lanes 4, 8, and 12). Presumed interstrand cross-links appear as low mobility bands.
Confirmation of Cross-Linking
In order to confirm that the low-mobility band corresponded to interstrand cross-linked DNA, we prepared Duplex B (Table 1), an oligomer similar to Duplex A except that the two strands differed in length. Each strand was independently radiolabeled and annealed to its cold complement. These duplexes were then incubated with ECH or EBH under the optimal reaction conditions, and the products were analyzed via dPAGE. The mobility of the presumed cross-link was similar in both cases (independent of whether the long or short strand was radiolabeled), supporting our assignment of the low-mobility band as an interstrand cross-link rather than a single-stranded product (Figure 3). Moreover, these low-mobility bands comigrated with the major low-mobility band in control DEB-cross-linked samples.
Figure 3.
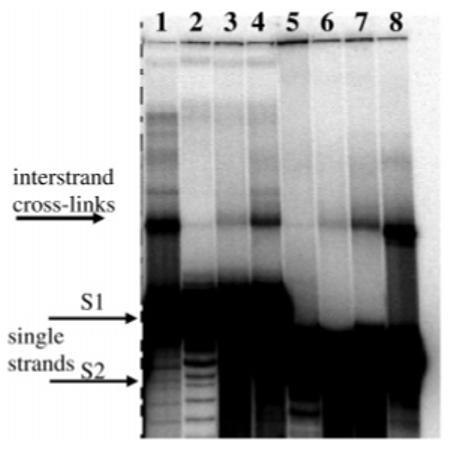
Cross-linked Duplex B, which has strands with different lengths (S1 is longer than S2). Lanes 1-4 are duplexes with S1 radiolabeled; lanes 5-8 are duplexes with S2 radiolabeled. Lanes 1 and 8, DEB (45 min, pH 7.0); lanes 2 and 5, control (no cross-linker); lanes 3 and 6, ECH (10 h, pH 5.0); lanes 4 and 7, EBH (4 h, pH 7.0).
Determination of Sequence Specificity
In order to examine the sequence specificity of cross-linking, we used Duplexes C, D, and E. These A/T-rich duplexes differed only in their central guanine-containing sequence, with Duplex C containing a central 5′-GC site, Duplex D, a central 5′-GGC site, and Duplex E, a central 5′-GGGC site (Table 1). Radiolabeled duplexes were independently incubated with ECH, EBH, or DEB (included for comparison) under optimal reaction conditions. Products were analyzed via dPAGE (Figure 4) and quantified via phosphorimagery (Table 2). Efficiencies of cross-linking followed the trend DEB > EBH > ECH. Furthermore, the cross-linking efficiencies of ECH and EBH did not vary significantly between the three duplexes, suggesting comparable cross-linking of 5′-GC and 5′-GGC sequences. However, DEB showed a 2-fold preference for the duplexes containing a central 5′-GGC site (D and E), as predicted by its established consensus sequence for cross-linking (7, 11).
Figure 4.
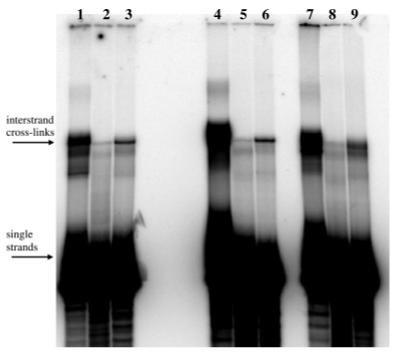
dPAGE analysis of the reactions of Duplexes C, D, and E. Lanes 1-3 are Duplex C; lanes 4-6 are Duplex D; and lanes 7-9 are Duplex E. Lanes 1, 4, and 7 are DEB products (45 min, pH 7.0); lanes 2, 5, and 8 are ECH products (10 h, pH 5.0); and lanes 3, 6, and 9 are EBH products (6 h, pH 7.0).
Table 2.
Average Percentages of Cross-Linking (% XL) of Duplex C (Containing a Central GC Site), Duplex D (Containing a Central GGC Site), and Duplex E (Containing a Central GGGC Site) with ECH, EBH, and DEBa
| ECH |
EBH |
DEB |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| duplex | C | D | E | C | D | E | C | D | E |
| average % XL | 1.2 | 1.8 | 0.87 | 5.4 | 4.6 | 6.6 | 14 | 30 | 27 |
| SD | 0.5 | 0.9 | 0.42 | 1.3 | 1.1 | 1.4 | 2 | 4 | 5 |
Data for a minimum of three replicate trials were averaged.
Determination of the Stereospecifity of ECH Cross-Linking
We examined the stereospecificity of ECH cross-linking by treating Duplex F, an A/T-rich duplex containing a central 5′-GGC site (Table 1), with R- or S-ECH. A previous report used mass spectrometry to confirm interstrand cross-linking of this duplex by DEB (39). As also observed by Tretyakova and co-workers (39), multiple bands of mobility lower than that of single strands were visible upon denaturing gel electrophoresis of this cross-linked duplex (Figure 5). Centrally cross-linked DNA has the lowest mobility on denaturing gels (40); therefore, we expected that the highest band corresponded to cross-linking at the 5′-GGC site. Tretyakova and co-workers also assigned this lowest-mobility band as the centrally cross-linked duplex (39). We independently confirmed this assignment through purification and piperidine cleavage. The predominant lower band was not piperidine-cleavable, suggesting that it corresponded to terminally cross-linked material, possibly between deoxyadenosine residues. However, the higher band produced cleavage products consistent with linkages between both deoxyguanosine residues on the top strand and the single deoxyguanosine on the bottom strand. Virtually no cross-link remained after piperidine treatment. The lowest-mobility product therefore corresponded to a mixture of the following cross-linked species, where the cross-linked residues are shown in bold.
Figure 5.
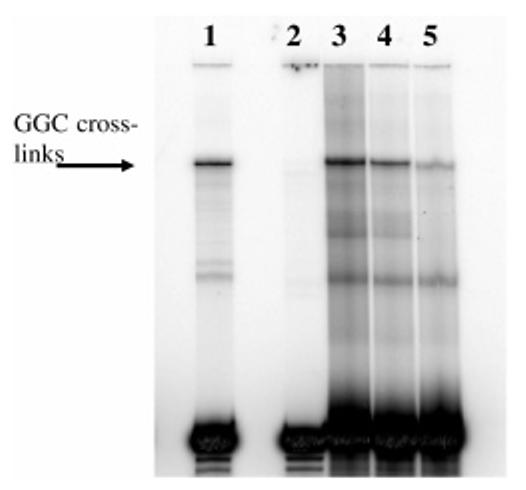
dPAGE analysis of the reaction of Duplex F with ECH optical isomers. Lane 1, DEB; lane 2, control (unmodified) DNA; lane 3, R-ECH; lane 4, racemic ECH; lane 5, S-ECH. Note that to compensate for the greater efficiency of DEB cross-linking, half as many counts of the DEB reaction was loaded compared to that for the ECH reactions. ECH reactions were 4 h and pH 5.0.
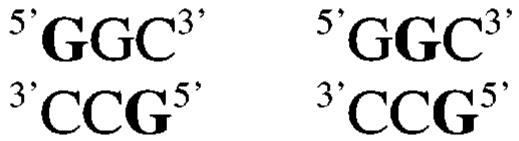
Quantitation of the lowest-mobility band revealed that the efficiency of cross-linking at the 5′-GGC site followed the order R-ECH > racemic ECH > S-ECH, with R-ECH about twice as efficient as S-ECH (Table 3).
Table 3.
Average Percentages of Cross-Linking (% XL) for Duplex F Treated with R-, S-, and Racemic ECH (4 h, pH 5.0)a
| cross-linker | R-ECH | S-ECH | racemic ECH |
|---|---|---|---|
| average % XL | 4.1 | 1.5 | 3.5 |
| SD | 0.9 | 0.4 | 0.9 |
Data for five replicate trials were averaged.
Determination of the Stereospecifity of ECH Cytotoxicity
In order to determine a possible relationship between interstrand cross-linking and cytotoxicity, the cytotoxicities of the two optical isomers of ECH were determined in 6C2 chicken erythroprogenitor cells. R-ECH was about twice as cytotoxic (IC50 = 2.2 mM ± (0.4) as S-ECH (IC50 = 3.9 mM ± (0.3), supporting a relationship between interstrand cross-linking and cytotoxicity. Although bifunctional alkylating agents react with a number of biological nucleophiles, reaction with DNA is generally considered most toxic (1). Furthermore, under the conditions used in these cytotoxicity experiments, only 11% of the 6C2 cells are in G1, the phase of the cell cycle expected to be the least susceptible to DNA-damaging agents (Table 4).
Table 4.
Average Percentages of 6C2 Cells in Various Phases of the Cell Cycle as Determined through Flow Cytometrya
| cell cycle phase | % population | SD |
|---|---|---|
| G1 | 11 | 0.2 |
| S | 2.8 | 0.4 |
| G2/M | 86 | 0.6 |
Data for three replicate trials were averaged.
Quantitation of Cross-Link Partitioning at the GGC Site
We also used Duplex F to confirm the reduced sequence specificity of the epihalohydrins relative to DEB. In particular, we wished to quantitate the partitioning of cross-links at the 5′-GGC versus the 5′-GC site for the epihalohydrins. We added a three-base (TAA) 5′-overhang to the bottom strand of Duplex F, allowing us to prepare duplexes that were either 3′-end labeled on the top strand, 5′-end labeled on the top strand, or 5′-end labeled on the bottom strand. We then used dPAGE to purify the lowest-mobility cross-linked product as well as monoadducts derived from duplexes in each of these radiolabeled states. Products were subjected to piperidine cleavage and analyzed via high-resolution sequencing gels and phosphorimagery to quantitate deoxyguanosine alkylation at the N7 position (38).
We averaged data for multiple 5′- and 3′-radiolabeling experiments of the top strand. This minimizes the effects of over-alkylation, which inflates the abundance of fragments closest to the radiolabeled end. That is, a monoalkylated guanine located between the bridged site and the radiolabeled end of the duplex masks the true site of cross-linking. However, multiple alkylation events would lead to the lower cleavage band always having the greater intensity no matter which end of the duplex was radiolabeled, which we did not observe in our experiments.
Our data confirmed the reduced sequence preferences of the epihalohydrins (Table 5) relative to DEB. The two deoxyguanosine residues within the 5′-GGC site were monoalkylated and cross-linked approximately equally by ECH and EBH. In contrast, the first deoxyguanosine residue was preferred 19-fold by DEB for cross-linking, although monoalkylation was approximately equal at the two residues. This is consistent with the established 5′-GNC consensus sequence for DEB cross-linking (7, 11). Note that cross-linking to the single central deoxyguanosine residue on the bottom strand was confirmed through complete piperidine cleavage of purified cross-link derived from the duplex that was 5′-radiolabeled on that strand.
Table 5.
Average Relative Percentages of Monoalkylation and Cross-Linking as a Function of Nucleotide Position within Duplex F (Containing a Central G1G2C Site)a
| ECH mono-alkylation |
ECH cross-linking |
EBH mono-alkylation |
EBH cross-linking |
DEB mono-alkylation |
DEB cross-linking |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nucleotide | avg% | SD | avg% | SD | avg% | SD | avg% | SD | avg% | SD | avg% | SD |
| G1 | 65 | 8 | 50 | 2 | 69 | 8 | 51 | 1 | 53 | 5 | 95 | 2 |
| G2 | 35 | 8 | 50 | 2 | 31 | 8 | 49 | 1 | 47 | 5 | 5 | 2 |
Relative intensities of piperidine cleavage products from separate experiments in which the top strand was either 5′- or 3′-end radiolabeled were averaged (a minimum of three replicate trials of each but equal numbers of trials for each end labeled strand).
Discussion
The formation of DNA interstrand cross-links has been demonstrated for many bifunctional electrophiles (1), including such potent anticancer agents as the nitrogen mustards, exemplified by HN2 (9, 10), and treosulfan, which is converted in vivo to DEB (41). Moreover, interstrand cross-linking has been found to correlate with mustard cytotoxicity (42). Although the probable carcinogen ECH is widely used as an industrial cross-linker, there have been no prior reports that it cross-links DNA. We screened for cross-linking with a duplex containing the 5′-GGCCC sequence preferred by DEB (11). Initial dPAGE analysis suggested only modest DNA cross-linking by ECH, leading us to include EBH, with its better leaving group, in our studies.
Optimizing reaction conditions for each of the epihalohydrins revealed that cross-linking efficiencies were highly dependent on pH, suggesting interesting mechanistic information about these reactions. In general, cross-linking proceeds via a two-step process: the formation of monoadducts and closure to cross-links (40). The mechanisms and relative rates of these steps vary by agent. For example, mustard cross-linking proceeds via an SN1 mechanism, with the rate-determining step corresponding to the formation of the aziridinium active intermediate (43). In contrast, DEB generally reacts via an SN2 mechanism with attack on the least substituted carbon in concert with ring opening (44, 45). The second epoxide ring then reacts readily, leading to relatively fast closure of monoadducts to cross-links (46).
In terms of epihalohydrin formation of monoadducts, the reaction could occur either by loss of the halide or by attack on the epoxide. Nucleophilic addition to ECH has been reported to occur initially at the epoxide ring (47). Indeed, the higher cross-linking efficiencies that we observed at pH 5.0 suggest that the ECH cross-linking reaction may be acid catalyzed, supporting the initial reaction of the epoxide to form monoadducts. This pathway is supported by the isolation of 7-(3-chloro-2-hydroxypropyl) guanine from calf thymus DNA (25, 31), whole rats (25), and human white blood cells (48) after exposure to ECH. In contrast, the lack of acid catalysis and the better leaving group suggest that epibromohydrin may react initially through the loss of the halide to form monoadducts. Furthermore, the reduced rate of EBH cross-linking under acidic conditions suggests that protonation of the oxirane slows the loss of bromide.
For many of the duplexes investigated, fairly diffuse or multiple low-mobility bands were formed by ECH and EBH, suggesting multiple products. Piperidine cleavage of the highest gel bands and subsequent analysis of the fragments supported linkage between N7 of deoxyguanosine residues. However, some low-mobility products were not cleaved by piperidine, such as for Duplex F, suggesting linkage at other sites as well. This observation is consistent with the “smeared” nature of the epihalohydrin-cross-linked bands as well as literature reports of deoxyadenosine adducts with calf thymus DNA (26). Residues near the ends of short duplexes have been noted to be hyperreactive toward mechlorethamine (10), DEB (7), and other agents considered to be somewhat flexible in their sequence specificity for cross-linking, such as formaldehyde (49), nitrous acid (50), and the pyrrolizidine alkaloids (51).
Overall, the epihalohydrins appear to be less stringent in their sequence requirements for cross-linking than either HN2 or DEB. Whereas HN2 and DEB have a relatively strong preference for the 5′-GNC sequence relative to 5′-GC, the epihalohydrins cross-linked both sites about equally. Interestingly, the 5′-GC sequence has the minimal N7-to-N7 interstrand distance, and was originally proposed to be the exclusive target of cross-linkers containing chains of five or fewer atoms (52). Other early reports proposed that cross-linking between N7 of guanine would be precluded for agents of less than seven carbon atoms in length (22). However, despite their short alkyl chains, ECH and EBH form interstrand cross-links between distal deoxyguanosine residues at 5′-GC and 5′-GGC sites about equally. Furthermore, monoalkylation is comparable for both deoxyguanosine residues within the 5′-GGC site. Sequence random monoalkylation has also been reported for both HN2 (40) and DEB (7), with cross-linking specificity arising during closure to cross-links. However, the antitumor agent mitomycin C has a strong preference for the monoalkylation of the 5′-CG sequence, which dictates its consensus sequence for cross-linking (53).
We also examined the role of stereospecificity in ECH cross-linking. The three optical isomers of DEB differ in their cytotoxicity, with S,S-DEB > R,R-DEB > meso-DEB (54-56). Furthermore, the interstrand cross-linking efficiencies of these compounds follow the same order (39), although the 5′-GNC consensus sequence is conserved (57). Our experiments showed that the two optical isomers of ECH also differed in both their interstrand cross-linking efficiencies and their cytotoxicities, with R-ECH about twice as effective as S-ECH. We used chicken 6C2 cells for the cytotoxicity studies because avian erythropoiesis is similar to that of mammals, and these erythroprogenitor cells are used to model the development of leukemia (58). Moreover, the use of DNA cross-linkers for cancer therapy is associated with the subsequent development of hemato-pathologies such as leukemia (2, 3).
The presumed monoalkylated intermediates differ for R- and S-ECH (Figure 6), suggesting different energies of the respective transition states for the cross-linking reaction. Although it is not clear whether the pathway from monoadducts to cross-links proceeds through direct displacement of the chloride or epoxide formation and subsequent ring opening, stabilizing interactions of the R- intermediate with neighboring groups may promote the formation of the interstrand cross-link. Characterization of the structures of the two 7-(3-chloro-2-hydroxypropyl) guanine monoalkylated intermediates could reveal the molecular basis for the different cross-linking efficiencies of the two ECH optical isomers.
Figure 6.
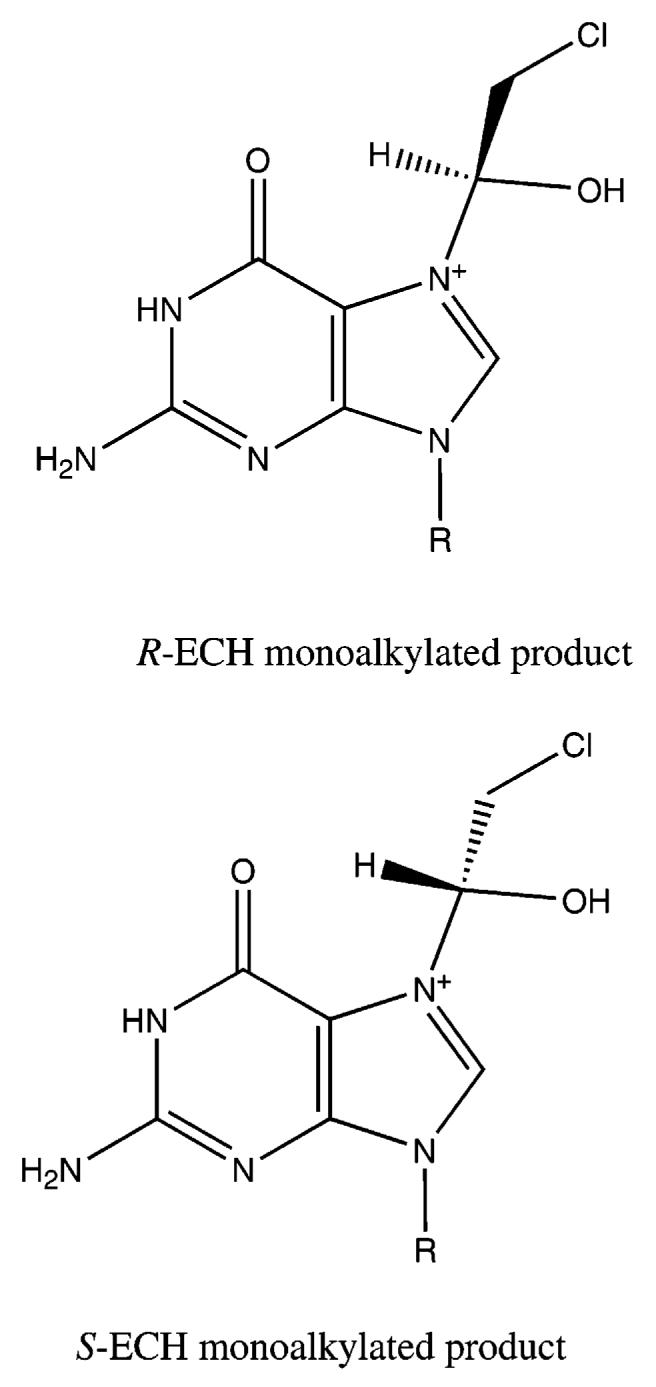
Proposed structures of the monoalkylated intermediates for R-ECH and S-ECH.
In conclusion, we have demonstrated that interstrand cross-linking between deoxyguanosine residues at 5′-GC and 5′-GGC sites occurs for ECH. Moreover, the correlation between cytotoxicity and cross-linking efficiency of the two optical isomers suggests that cross-linking may play a role in the adverse biological effects of this important industrial compound.
Acknowledgment
We thank Andrea Bassi, Tiffany Frazar, Scott Whitlow, and Junko Goda for preliminary work. We also thank Professors Paul Greenwood, Jeffrey Katz, Fred LaRiviere, Brad Mundy, and Kevin Rice for helpful discussions and Dr. David Bodine for 6C2 cells. This work was supported by NIH Academic Research Enhancement Award R15 CA077748 from the National Cancer Institute, the Donors of the American Chemical Society Petroleum Research Fund (PRF# 44839-B4), a Henry Dreyfus Teacher-Scholar Award, and NIH Grant Number P20 RR-016463 from the INBRE Program of the National Center for Research Resources.
Footnotes
- DEB
- diepoxybutane
- HN2
- mechlorethamine
- ECH
- epichlorohydrin
- EBH
- epibromohydrin
- TE
- 10 mM Tris buffer and 1 mM EDTA (pH 7.0)
- MES
- 2-(N-morpholino)ethanesulfonic acid
- dPAGE
- denaturing polyacrylamide gel electrophoresis
- bp
- base pairs.
References
- (1).Rajski SR, Williams RM. DNA cross-linking agents as antitumor drugs. Chem. Rev. 1998;98:2723–2795. doi: 10.1021/cr9800199. [DOI] [PubMed] [Google Scholar]
- (2).Kaldor JM, Day NE, Clarke EA, Van Leeuwen FE, Henry-Amar M, Fiorentino MV, Bell J, Pedersen D, Band P, Assouline D, Koch M, Choi W, Prior P, Blair V, Langmark F, Kirn VP, Neal F, Peters D, Pfeiffer R, Karjalainen S, Cuzick J, Sutcliffe SB, Somers R, Pellae-Cosset B, Pappagallo GL, Fraser P, Storm H, Stovall M. Leukemia risk following Hodgkin’s disease. N. Engl. J. Med. 1990;322:7–13. doi: 10.1056/NEJM199001043220102. [DOI] [PubMed] [Google Scholar]
- (3).Van Leeuwen FE, Chorus AM, van den Belt-Dusebout AW, Hagenbeek A, Noyon R, van Kerkhoff EH, Pinedo HM, Somers R. Leukemia risk following Hodgkin’s disease: relation to cumulative dose of alkylating agents, treatment with teniposide combinations, number of episodes of chemotherapy, and bone marrow damage. J. Clin. Oncol. 1994;12:1063–1073. doi: 10.1200/JCO.1994.12.5.1063. [DOI] [PubMed] [Google Scholar]
- (4).Divine BJ. An update on mortality among workers at a 1,3-butadiene facility. Environ. Health Perspect. 1990;86:119–128. doi: 10.1289/ehp.9086119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Divine BJ, Wendt JK, Hartman CM. Cancer Mortality among Workers at a Butadiene Production Facility. In: Sorsa M, Peltonen K, Vainio H, Hemminki K, editors. Butadiene and Styrene: Assessment of Health Hazards. IARC; Lyon, France: 1993. pp. 345–362. IARC Scientific Publications No. 127. [PubMed] [Google Scholar]
- (6).Macaluso M, Larson R, Delzell E, Sathiakumar N, Hovinga M, Julian J, Muir D, Cole P. Leukemia and cumulative exposure to butadiene, styrene, and benzene among workers in the synthetic rubber industry. Toxicology. 1996;113:190–202. doi: 10.1016/0300-483x(96)03444-0. [DOI] [PubMed] [Google Scholar]
- (7).Millard JT, White MM. Diepoxybutane cross-links DNA at 5′-GNC sequences. Biochemistry. 1993;32:2120–2124. doi: 10.1021/bi00059a034. [DOI] [PubMed] [Google Scholar]
- (8).Millard JT, Wilkes EE. Diepoxybutane and diepoxyoctane interstrand cross-linking of the 5S DNA nucleosomal core particle. Biochemistry. 2001;40:10677–10685. doi: 10.1021/bi0109663. [DOI] [PubMed] [Google Scholar]
- (9).Ojwang JO, Grueneberg DA, Loechler EL. Synthesis of a duplex oligonucleotide containing a nitrogen mustard interstrand DNA-DNA cross-link. Cancer Res. 1989;49:6529–6537. [PubMed] [Google Scholar]
- (10).Millard JT, Raucher S, Hopkins PB. Mechlorethamine cross-links deoxyguanosine residues at 5′-GNC sequences in duplex DNA fragments. J. Am. Chem. Soc. 1990;112:2459–2460. [Google Scholar]
- (11).Sawyer GA, Frederick ED, Millard JT. Flanking sequences modulate diepoxide and mustard cross-linking efficiencies at the 5′-GNC site. Chem. Res. Toxicol. 2004;17:1057–1063. doi: 10.1021/tx0499057. [DOI] [PubMed] [Google Scholar]
- (12).Camara Grenier EO, Kälin T, Yoneyama M. Chemical Industries Newsletter. SRI Consulting; Menlo Park, CA: Sep, 2004. [accessed February 2007]. Chemical Economics Handbook Product Review Abstract: Epichlorohydrin; pp. 5–6. 2004. www.sriconsulting.com/nl/Public/2004Sep.pdf; [Google Scholar]
- (13).IARC . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 71. Lyon, France: 1999. Epichlorohydrin; pp. 603–628. [PMC free article] [PubMed] [Google Scholar]
- (14).Kucerová M, Zhurkov VS, Polívková Z, Ivanova JE. Mutagenic effect of epichlorohydrin. II. Analysis of chromosomal aberrations in lymphocytes of persons occupationally exposed to epichlorohydrin. Mutat. Res. 1977;48:355–360. doi: 10.1016/0027-5107(77)90178-6. [DOI] [PubMed] [Google Scholar]
- (15).Picciano D. Cytogenetic investigation of occupational exposure to epichlorohydrin. Mutat. Res. 1979;66:169–173. doi: 10.1016/0165-1218(79)90062-4. [DOI] [PubMed] [Google Scholar]
- (16).Kolman A, Chovanec M, Osterman-Golkar S. Genotoxic effects of ethylene oxide, propylene oxide and epichlorohydrin in humans: update review (1990-2001) Mutat. Res. 2002;512:173–194. doi: 10.1016/s1383-5742(02)00067-4. [DOI] [PubMed] [Google Scholar]
- (17).Perocco P, Rocchi P, Ferreri AM, Capucci A. Toxic, DNA-damaging and mutagenic activity of epicholorohydrin on human cells cultured in vitro. Tumori. 1983;69:191–194. doi: 10.1177/030089168306900303. [DOI] [PubMed] [Google Scholar]
- (18).Giri AK. Genetic toxicology of epicholorohydrin. Mutat. Res. 1997;386:25–38. doi: 10.1016/s1383-5742(96)00042-7. [DOI] [PubMed] [Google Scholar]
- (19).Kolman A, Dusinská M. Comparison of propylene oxide and epichlorohydrin effects in two transformation tests (C3H/10T1/2 and SHE cells) Toxicol. Lett. 1995;81:213–221. doi: 10.1016/0378-4274(95)03442-0. [DOI] [PubMed] [Google Scholar]
- (20).Laskin S, Sellakumar AR, Kuschner M, Nelson N, La Mendola S, Rusch GM, Katz GV, Dulak NC, Albert RE. Inhalation carcinogenicity of epichlorohydrin in noninbred Sprague-Dawley rats. J. Natl. Cancer Inst. 1980;65:751–755. doi: 10.1093/jnci/65.4.751. [DOI] [PubMed] [Google Scholar]
- (21).Hans B, Kaur S, Sangha GK. Epichlorohydrin induced biochemical changes in the rose-ringed parakeet Psittacula krameri scopoli. Ind. J. Exp. Biol. 1999;37:774–777. [PubMed] [Google Scholar]
- (22).Van, Duuren BL, Goldschmidt BM. Carcinogenicity of epoxides, lactones, and peroxy compounds. III. Biological activity and reactivity. J. Med. Chem. 1966;9:77–79. doi: 10.1021/jm00319a020. [DOI] [PubMed] [Google Scholar]
- (23).Mazzullo M, Colacci A, Grilli S, Prodi G, Arfellini G. In vivo and in vitro binding of epichlorohydrin to nucleic acids. Cancer Lett. 1984;23:81–90. doi: 10.1016/0304-3835(84)90065-x. [DOI] [PubMed] [Google Scholar]
- (24).Hemminki K. Fluorescence study of DNA alkylation by epoxides. Chem.-Biol. Interact. 1979;28:269–278. doi: 10.1016/0009-2797(79)90167-4. [DOI] [PubMed] [Google Scholar]
- (25).Landin HH, Segerbäck D, Damberg C, Osterman-Golkar Adducts with haemoglobin and with DNA in epichlorohydrin-exposed rats. Chem.-Biol. Interact. 1999;117:49–64. doi: 10.1016/s0009-2797(98)00099-4. [DOI] [PubMed] [Google Scholar]
- (26).Sund P, Kronberg L. Reaction of epichlorohydrin with adenosine, 2′-deoxyadenosine and calf thymus DNA: Identification of adducts. Bioorg. Chem. 2006;34:115–130. doi: 10.1016/j.bioorg.2006.01.005. [DOI] [PubMed] [Google Scholar]
- (27).Singh G, Hauswirth WW, Ross WE, Neims AH. A method for assessing damage to mitochondrial DNA caused by radiation and epichlorohydrin. Mol. Pharmacol. 1985;27:167–170. [PubMed] [Google Scholar]
- (28).Kolman A, Spivak I, Näslund M, Dusinská M, Cedervall B. Propylene oxide and epichlorohydrin induce DNA strand breaks in human diploid fibroblasts. Environ. Mol. Mutagen. 1997;30:40–46. doi: 10.1002/(sici)1098-2280(1997)30:1<40::aid-em6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- (29).Hemminki K, Paasivirta J, Kurkirinne T, Virkki L. Alkylation of DNA bases by simple epoxides. Chem.-Biol. Interact. 1980;30:259–270. doi: 10.1016/0009-2797(80)90049-6. [DOI] [PubMed] [Google Scholar]
- (30).Djuric Z, Hooberman BH, Rosman L, Sinsheimer JE. Reactivity of mutagenic propylene oxides with deoxynucleosides and DNA. Environ. Mutagen. 1986;8:369–383. doi: 10.1002/em.2860080306. [DOI] [PubMed] [Google Scholar]
- (31).Singh US, Decker-Samuelian K, Solomon JJ. Reaction of epichlorohydrin with 2′-deoxynucleosides: characterization of adducts. Chem.-Biol. Interact. 1996;99:109–128. doi: 10.1016/0009-2797(95)03665-2. [DOI] [PubMed] [Google Scholar]
- (32).Mäki J, Karlsson K, Sjöholm R, Kronberg L. Structural characterisation of the main epichlorohydrin-guanosine adducts. Adv. Exp. Med. Biol. 2001;500:125–128. [PubMed] [Google Scholar]
- (33).He Y, Nagano M, Yamamoto H, Miyamoto E, Futatsuka M. Modifications of neurofilament proteins by possible metabolites of allyl chloride in vitro. Drug Chem. Toxicol. 1995;18:315–331. doi: 10.3109/01480549509014326. [DOI] [PubMed] [Google Scholar]
- (34).Voogd CE, van der Stel JJ, Jacobs J. The mutagenic action of aliphatic epoxides. Mutat. Res. 1981;89:269–282. doi: 10.1016/0165-1218(81)90108-7. [DOI] [PubMed] [Google Scholar]
- (35).Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- (36).Freshney RI. Culture of Animal Cells: A Manual of Basic Techniques. Wiley-Liss; Hoboken, NJ: 2005. [Google Scholar]
- (37).Darzynkiewicz Z, Juan G, Bedner E. Determining cell cycle stages by flow cytometry. Curr. Protoc. Cell Biol. 1999;8:1–18. doi: 10.1002/0471143030.cb0804s01. [DOI] [PubMed] [Google Scholar]
- (38).Luce RA, Hopkins PB. Chemical cross-linking of drugs to DNA. Methods Enzymol. 2001;340:396–412. doi: 10.1016/s0076-6879(01)40433-2. [DOI] [PubMed] [Google Scholar]
- (39).Park S, Anderson C, Loeber R, Jones R, Tretyakova N. Interstrand and intrastrand DNA-DNA cross-linking by 1,2,3,4-diepoxybutane: role of stereochemistry. J. Am. Chem. Soc. 2005;127:14355–14365. doi: 10.1021/ja051979x. [DOI] [PubMed] [Google Scholar]
- (40).Hopkins PB, Millard JT, Woo J, Weidner MF, Kirchner JJ, Sigurdsson ST, Raucher S. Sequence preferences of DNA interstrand cross-linking agents: importance of minimal DNA structural reorganization in the cross-linking reactions of mechlore-thamine, cisplatin, and mitomycin C. Tetrahedron. 1991;47:2475–2489. [Google Scholar]
- (41).Hartley JA, O’Hare CC, Baumgart J. DNA alkylation and interstrand cross-linking by treosulfan. Br. J. Cancer. 1999;79:264–266. doi: 10.1038/sj.bjc.6690043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Sunters A, Springers CJ, Bagshawe KD, Souhami RL, Hartley JA. The cytotoxicity, DNA crosslinking ability and DNA sequence selectivity of the aniline mustards melphalan, chloram-bucil, and 4-[bis](2-chloroethyl)amino] benzoic acid. Biochem. Pharmacol. 1992;44:59–64. doi: 10.1016/0006-2952(92)90038-k. [DOI] [PubMed] [Google Scholar]
- (43).Rutman RJ, Chun EHL, Jones J. Observations on the mechanism of the alkyation reaction between nitrogen mustard and DNA. Biochim. Biophys. Acta. 1969;174:663–673. doi: 10.1016/0005-2787(69)90295-0. [DOI] [PubMed] [Google Scholar]
- (44).Ross WCJ. The chemistry of cytotoxic alkylating agents. Adv. Cancer Res. 1953;1:397–449. doi: 10.1016/s0065-230x(08)60008-1. [DOI] [PubMed] [Google Scholar]
- (45).Ehrenberg L, Hussain S. Genetic toxicity of some important epoxides. Mutat. Res. 1981;86:1–113. doi: 10.1016/0165-1110(81)90034-8. [DOI] [PubMed] [Google Scholar]
- (46).Ross WCJ. The reaction of certain epoxides in aqueous solutions. J. Chem. Soc. 1950:2257–2272. [Google Scholar]
- (47).McClure DE, Arison BH, Baldwin JJ. Mode of nucleophilic addition to epichlorohydrin and related species: chiral aryloxymethyloxiranes. J. Am. Chem. Soc. 1979;101:3666–3668. [Google Scholar]
- (48).Plna K, Osterman-Golkar S, Nogradi E, Segerbäck D. 32P-post-labelling of 7-(3-chloro-2-hydroxypropyl)guanine in white blood cells of workers occupationally exposed to epichlorohydrin. Carcinogenesis. 2000;21:275–280. doi: 10.1093/carcin/21.2.275. [DOI] [PubMed] [Google Scholar]
- (49).Huang H, Hopkins PB. DNA interstrand cross-linking by formaldehyde: nucleotide sequence preference and covalent structure of the predominant cross-link formed in synthetic oligo-nucleotides. J. Am. Chem. Soc. 1993;115:9402–9408. [Google Scholar]
- (50).Kirchner JJ, Hopkins PB. Nitrous acid cross-links duplex DNA fragments through deoxyguanosine residues at the sequence 5′-CG. J. Am. Chem. Soc. 1991;113:4681–4682. [Google Scholar]
- (51).Weidner MF, Sigurdsson S. Th., Hopkins PB. Sequence preferences of DNA interstrand cross-linking agents: dG-to-dG cross-linking at 5′-GC by structurally simplified analogues of mitomycin C. Biochemistry. 1990;29:9225–9233. doi: 10.1021/bi00491a017. [DOI] [PubMed] [Google Scholar]
- (52).Brookes P, Lawley PD. The reaction of mono- and di-functional alkylating agents with nucleic acids. Biochem. J. 1961;80:496–503. doi: 10.1042/bj0800496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Kumar S, Lipman R, Tomasz M. Recognition of specific DNA sequences by mitomycin C for alkylation. Biochemistry. 1992;31:1399–1407. doi: 10.1021/bi00120a016. [DOI] [PubMed] [Google Scholar]
- (54).Verly WG, Brakier L, Feit PW. Inactivation of the T7 coliphage by the diepoxybutane stereoisomers. Biochim. Biophys. Acta. 1971;228:400–406. doi: 10.1016/0005-2787(71)90046-3. [DOI] [PubMed] [Google Scholar]
- (55).Matagne R. Induction of chromosomal aberrations and mutations with isomeric forms of L-threitol-1,4-bismethanesulfonate in plant materials. Mutat. Res. 1969;7:241–247. doi: 10.1016/0027-5107(69)90037-2. [DOI] [PubMed] [Google Scholar]
- (56).Bianchi A, Contin M. Mutagenic activity of isomeric forms of diepoxybutane in maize. J. Hered. 1962;53:277–281. [Google Scholar]
- (57).Millard JT, Hanly TC, Murphy K, Tretyakova N. The 5′-GNC site for DNA interstrand cross-linking is conserved for diepoxybutane stereoisomers. Chem. Res. Toxicol. 2006;19:16–19. doi: 10.1021/tx050250z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Robinson D, Chen HC, Li D, Yustein JT, He F, Lin WC, Hayman MJ, Kung HJ. Tyrosine kinase expression profiles of chicken erythro-progenitor cells and oncogene-transformed erythroblasts. J. Biomed. Sci. 1998;5:93–100. doi: 10.1007/BF02258362. [DOI] [PubMed] [Google Scholar]


