Table 1.
 | ||||
|---|---|---|---|---|
| X = OMe a 0% b 0% |
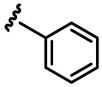 a 3% b 0% |
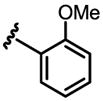 a 0% b 0% |
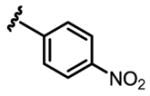 a 12% b 0% |
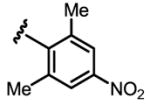 a 10% b 0% |
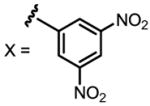 a 24% b 12% |
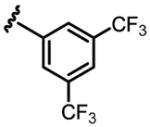 a 18% b 8% |
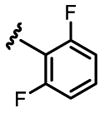 a 30% b 22% |
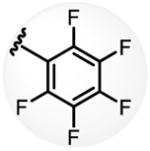 a 34% b 54% |
|
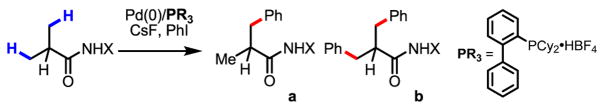
Conditions: 0.2 mmol of substrate, 10 mol% Pd(OAc)2, 20 mol% ligand, 3.0 equiv of CsF, 3.0 equiv of aryl iodide, 100 mg 3Å MS, 1 mL toluene, 100 °C, N2, 24 h.
Yield was determined by 1H NMR analysis of crude product using CH2Br2 as the internal standard.
