Introduction
Established renal failure (ERF) in children and young adults is considered a rare disease. Single country data may be underpowered to show any differences or effects of treatment and therefore cooperation between countries is essential to improve the outcome of children with ERF. Until 2007 data collection on children and young adults on renal replacement therapy (RRT) in Europe was limited to that of the European Renal Association–European Dialysis and Transplant (ERA–EDTA) Registry. Within the ERA–EDTA Registry data are collected from national and regional renal registries. As these do not always include paediatric patients, data on children have only been available from a limited part of Europe. The first publication on paediatric patients by the Amsterdam-based ERA–EDTA Registry included data on 3184 patients (aged 0–19 years) from 12 registries in 11 countries, covering a total general population of 80.3 million, who started RRT between 1980 and 2000 [1]. Information was presented on incidence, prevalence, primary renal disease, age at onset of RRT, type of treatment and survival. The data demonstrated an almost 3-fold increase in the prevalence of ERF over 20 years, from 22.9 per million age related population (pmarp) in 1980 to 62.1 pmarp in 2000. The incidence rose from 7.1 pmarp in the 1980–84 cohorts to 9.9 pmarp in the 1985–89 cohorts but then remained stable. In the early cohort haemodialysis was the most common form of first treatment modality, but by 1995–2000 peritoneal dialysis and pre-emptive transplant were increasingly used. Over the 20 years studied the relative risk of death for patients starting dialysis or following their first transplant reduced by 36% and 42%, respectively.
A paper on the characteristics and survival of young adults who started RRT during childhood has recently been published [2], and a further study on the effect of timing of transplantation is in progress. In addition, an international study comparing survival between children on haemodialysis and peritoneal dialysis is running in collaboration with other large registries including the USRDS and ANZDATA [3].
Since 2004 preparations have been made for a more comprehensive registry specifically focusing on children, the European Society for Paediatric Nephrology (ESPN)/ERA–EDTA Registry. It was decided that the data collection on children should be expanded not only to include more countries but also to collect much more detailed data on different aspects of ERF management. Initially, established registries from the larger countries were compared to see if a common database could be developed. A paediatric sub-committee of the ERA–EDTA Registry committee was set up to further develop the paediatric aspects of the Registry. This led to a comparison of UK and Italian data that was presented at the ERA–EDTA meeting in Lisbon in ‘Auditing and Advancing Paediatric RRT’. This comparison demonstrated that these two countries not only had very similar populations of children with ERF, but there was also evidence of differences in management, for example of haemodialysis access and time to transplantation. In 2005, with the inclusion of patients from the UK, Italy and Turkey, the total number of incident patients under the age of 15 reported to the ERA–EDTA Registry increased from 2009 to 4280 and details were reported at the ESPN meeting in Istanbul 2005 in ‘The new paediatric EDTA database’.
At the same time the ERA–EDTA Registry set out to develop the QUEST (QUality European STudies) initiative [4]. The aim of this initiative is to identify comparable indicators to assess the quality of RRT care among European countries, with the aim of improving outcome across Europe. One of the ways of reaching that objective is to develop and standardize clinical performance indicators to be used for (inter)national benchmarking. Together with national renal registries and societies the ERA–EDTA Registry applied to the European Union for a grant, under the name of NephroQUEST, which was subsequently awarded in 2007. The project was officially launched at a meeting in Amsterdam in November 2007 [5]. It is hoped that this project will support the development of the infrastructure required for data collection in countries without this facility that will then be of benefit to both children and adults with renal failure. The NephroQUEST grant has also provided co-funding for the employment of a part-time dedicated epidemiologist, for the paediatric registry, for 3 years. Contributions to the funding for this post have also been provided by the ERA–EDTA and the ESPN for this period and action is now required to secure long-term funding.
The launch of the ESPN/ERA-EDTA Registry
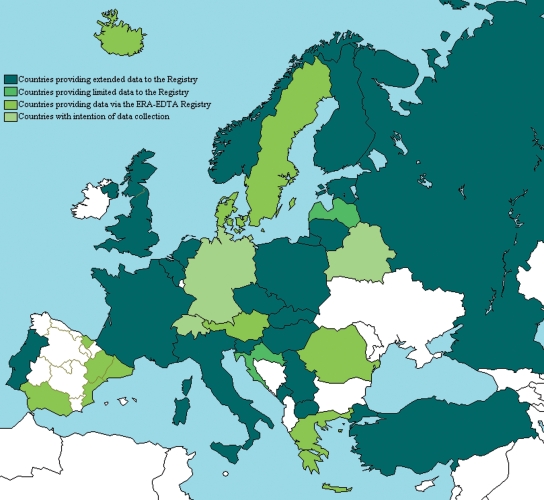
In 2007 the combined ESPN/ERA–EDTA Registry was officially launched. An epidemiologist was appointed to work with the AMC-based ERA–EDTA Registry and the two ESPN representatives with the ERA–EDTA Registry committee, in order to develop the separate paediatric aspects of the Registry. Forty European countries were contacted via the presidents of national societies or known registry leads and 35 responded favourably to being interested in participating in the future registry. Since then 33 paediatric registries (29 countries) have contributed data (Figure 1). As of September 2008 the Registry had collected data from countries covering a total general population of ∼400 million individuals.
Fig. 1.
Countries contributing data to the ESPN/ERA–EDTA Registry.
To facilitate data collection, particularly for those countries without established registries, a new paediatric database has been developed which has been distributed to all European countries. Individual patient data will be collected on all patients starting RRT. The database has been sent to the national representatives who are responsible for the data collection. They can decide to send data from their national registry to the ESPN/ERA–EDTA Registry directly, import the data from their registry into the ESPN/ERA–EDTA database or ask the different centres to provide the individual patient data directly into the database. Although a significant amount of data will be collected on all patients, the database also allows identification of specific cohorts that might be the subject of more in depth analysis as part of a separate research project.
The data collection is structured into the ERA–EDTA core dataset (essential) including date of birth, gender, cause of renal disease, date of first RRT, treatment modality, changes of therapy, death and its cause and transfer to other countries and two extended datasets (based on NephroQUEST datasets adapted for children); the first extension ‘A’ including high priority data collection and the second extension ‘B’ with medium priority data collection. These extended datasets will enable the registry to collect more detailed information from cohorts of patients in order to study more specific areas.
Currently 24/29 of the countries included are 100% complete with respect to patients for their coverage area and 25/29 cover both dialysis and transplant patients. The level of detail of data collection differs by country, some countries collecting the minimum dataset of 9 variables and others of >50 variables. However, it is hoped that the new database will improve the level of detail and the standardization of data collection for all countries.
In the future, if quality is sufficient, and the coverage could be guaranteed, it is hoped that the data collection will expand to include those with pre-end-stage renal failure.
Future plans for the ESPN/ERA-EDTA Registry
Whereas different projects and related papers on paediatric ESRF are in progress within the ERA–EDTA Registry, the more extensive data collection within the Registry will enable more specific research projects to be developed.
The Registry provides the opportunity to study significant numbers of children with rare causes of renal failure. For example, haemolytic uraemic syndrome (HUS), particularly atypical HUS, is a rare cause of renal failure overall (accounting for 2.9% of patients with ERF [6]). Combining experience from the whole of Europe will allow improved statistical power and therefore more meaningful analysis of the data. This Registry can also be used as a sampling frame to identify cohorts of children in whom even more detailed research could be undertaken. Areas that have already been identified for detailed study include growth, nutrition, cardiovascular morbidity and risk factors, RRT in infants and specific conditions such as management of focal segmental glomerulosclerosis or HUS in the post-transplant period.
Two further issues are important in studying paediatric RRT. Firstly, as a large proportion of the children have a transplant, a strong collaboration between transplant and dialysis registries is essential. It is recommended that the two should be merged into one RRT registry to ensure that the entire therapy history from all children is available. Secondly, in paediatrics the improvement in care requires the knowledge of not only the short-term outcome but also of the long-term outcome of the patients who have received RRT in childhood, in terms of survival, comorbidity and treatment-related complications. For this reason it is essential that paediatric registries develop seamless collaboration, in data collection and reporting, with registries only collecting data on adult patients. Therefore, all national registries, the paediatric as well as those collecting adult data, are encouraged to develop these links in the near future.
Physicians, researchers and others interested in research in paediatric nephrology are highly encouraged to suggest research questions, initiate projects within the ESPN or to visit the ESPN/ERA-EDTA Registry for an internship in paediatric RRT research.
Acknowledgments
We would sincerely like to thank the members of the ESPN/ERA-EDTA Registry scientific sub-committee JW Groothoff and M Lewis and of the ESPN/ERA-EDTA Registry committee C Holmberg, P Cochat, R Coppo, P Hoyer and P Niaudet. Furthermore, we would like to thank the patients and staff of all dialysis and transplant units who have contributed data via their national and regional registries. We are also indebted to all the countries and registry leads and those who contributed all data and cooperated with the ESPN Registry namely those from Austria (R Kramar and R Oberbauer), Belarus (A Sukalo and I Kazyra), Belgium (F Janssen, R van Damme-Lombaerts and F Collart), Croatia (D Batinić and Z Puretic), Czech Republic (T Seeman and K Vondrak), Denmark (J Heaf), Estonia (M Ots, I Vainumäe and A Traat), Finland (P Finne C Grönhagen-Riska and C Holmberg), France (P Niaudet and L le Mignot), Former Yugoslavian Republic of Macedonia (E Sahpazova, N Bojkovska and D Kuzmanovska), Germany (C Barth, F Schaefer and P Hoyer), Greece (GA Ioannidis and D Stefandis), Hungary (G Reusz, L Szabo and T Szabo), Iceland (R Palsson), Israel (Y Frishberg), Italy (E Verrina, DA Mattucci and S Varriale), Latvia (V Strazdins), Lithuania (A Jankauskiene), Montenegro (S Pavićević), Norway (T Leivestad), Poland (A Zurowska and I Zagozdzon), Portugal (H Jardim and C Mota), Romania (L Garneata, M Gafencu and G Mircescu), Russia (E Molchanova and BT Bikbov), Republic of Serbia (A Peco-Antic, D Paripovic, and M Kostic), Slovakia (L Podracka and D Kolvek), Slovenia (J Buturović-Ponikvar, G Novljan and RB Kenda), Andalusia, Spain (P Castro), Aragon, Spain (Registro de Insuficiencia Renal Crónica en Tratamiento Sustitutivo de Aragón), Basque country, Spain (R Alonso), Catalonia, Spain (Á Magaz, J Aranzabal, I Lampreabe and J Arrieta), Valencian Region, Spain (O Zurriaga and MJ Garcia-Blasco †), Sweden (S Schön, J Ahlmén, and M Herthelius), Switzerland (CE Kuehni and E Maurer), the Netherlands (F Scheelings, D Vandic and J Groothoff), Turkey (R Topaloglu), UK (M Lewis). The ESPN/ERA–EDTA Registry is funded by the European Society of Paediatric Nephrology (ESPN), the European Renal Association (ERA–EDTA) and the NephroQUEST project. The NephroQUEST project has received funding from the European Union in the framework of the Public Health Programme. Furthermore, Amgen has agreed to provide an unrestricted grant to assist the ESPN in the financial support of the Registry.
Conflict of interest statement. None declared.
References
- 1.Van Der Heijden BJ, van Dijk PCW, Verrier-Jones K, et al. Renal replacement therapy in Children: data from 12 registries in Europe. Pediatr Nephrol. 2004;19:213–221. doi: 10.1007/s00467-003-1376-x. [DOI] [PubMed] [Google Scholar]
- 2.Kramer A, Stel VS, Tizard EJ, et al. Characteristics and survival of young adults who started renal replacement therapy during childhood. Nephrol Dial Transplant. 2009;24:926–933. doi: 10.1093/ndt/gfn542. [DOI] [PubMed] [Google Scholar]
- 3.McDonald SP, Craig J, Agodoa LY, et al. Dialysis outcomes among pediatric patients by dialysis modality—a multinational registry study. J Am Soc Nephrol. 2007;18:729A. [Google Scholar]
- 4.Jager KJ, Zoccali C. QUality European STudies (QUEST)—a step forward in the quality of RRT care. Nephrol Dial Transplant. 2005;20:2005–2006. doi: 10.1093/ndt/gfi036. [DOI] [PubMed] [Google Scholar]
- 5.Jager KJ, Zoccali C. Quality of care in end-stage renal disease: the importance of comparing ‘apples with apples’. Nephrol Dial Transplant. 2008;23:1116. doi: 10.1093/ndt/gfm876. [DOI] [PubMed] [Google Scholar]
- 6.ERA-EDTA registry ERA-EDTA Registry 2006 Annual Report. Department of Medical Informatics, Academic Medical Center, Amsterdam, The Netherlands, 2008; Information on the ESPN Registry can be found on the registry website http://www.espn-reg.org .