Abstract
We present a patient with right-hemispheric speech lateralization who exhibited severe recognition and naming deficits for unique objects (famous faces and landmarks) and grossly normal recognition and naming performances for nonunique objects (animals and man-made objects) following an anterior right temporal lobe (TL) resection of a ganglioglioma. While recognition deficits have been reported for famous faces following right temporal pole lesions, and for landmarks and geographic regions following right TL damage in general, this is the first reported case of both recognition and naming deficits for these objects resulting from a single lesion. These results are consistent with research suggesting that the neuroanatomic substrates for the recognition and naming of unique objects lie in the anterior TL regions. Left temporal pole lesions have been associated with naming deficits for unique objects while right temporal pole lesions have been associated with recognition deficits for unique objects. However, these findings suggest that the substrates of naming can be located in homotopic regions of the right hemisphere when language lateralization is atypical. As various object categories appear to have different neuroanatomical representations in the TLs, we discuss the possible benefits of sampling a wider array of objects during cortical stimulation mapping of language.
Keywords: Semantic memory, Category-specific naming and recognition deficits, Temporal lobes, Tumor resection, Atypical speech lateralization
Category-specific recognition and naming deficits have been observed in a variety of patient populations, including HSV encephalitis, semantic dementia, Alzheimer’s disease, and stroke (Chan et al., 2001; Laws and Sartori, 2005; Damasio et al., 1996; Snowden et al., 2004). Such deficits represent an inability to either recognize or to name one or more select categories of objects although general language and visuo-perceptual functions remain grossly intact. We present a patient with right hemisphere speech dominance who exhibited both naming and recognition deficits for unique objects following the resection of a right temporal lobe (TL) tumor.
Both lesion analytic studies and functional neuroimaging paradigms have highlighted the importance of the TLs in the recognition and naming of several object categories (Damasio et al., 1996; Koenig et al., 2005; Grabowski et al., 2001; Warrington and Shallice, 1984). Lesions in the left anterior TL have often been associated with category-specific naming deficits involving unique objects such as famous faces or landmarks, while more posterior left TL lesions have been associated with impaired naming of man-made objects (Damasio et al., 1996; Tranel, 2006). Some evidence also suggests that lesions involving the right temporal pole are associated with impaired recognition of unique objects such as famous faces (Glosser et al., 2003), and that lesions of the right TL in general may affect recognition of familiar surroundings or famous places (Landis et al., 1986). Tranel et al. (1997a, 1997b) found a selective deficit involving the recognition of animals associated with right occipital lobe lesions with extension into the right mesial inferior TL. This group also showed that recognition deficits for man-made objects occurred with posterior left TL lesions. Although we have focused only on the TL sites that seem to play a role in object recognition and naming, other brain regions (e.g., occipital lobe) may also be important (Damasio et al., 2004; Barsalou et al., 2003).
In most cases of category-specific deficits resulting from a single lesion, patients experience a disruption of either naming or recognition of one or more subsets of items but not both of these cognitive processes (Damasio et al., 1996; Tranel et al., 1997a, 1997b). For example, a person exhibiting naming deficits for one or more categories (e.g., animals, famous persons, landmarks, fruits) will continue to recognize the objects from these categories. Similarly, someone exhibiting category-specific recognition deficits will typically be able to name the objects from these deficient categories that they remain able to recognize. The one consistent exception to this pattern has been category-specific deficits for man-made objects, which can include both recognition and naming deficits resulting from a single lesion. Such patients will demonstrate a general problem with recognition for a specific category, but will also have difficulty in naming the objects that they are able to recognize. For someone with recognition deficits, we can continue to assess naming performance by examining their ability to name those objects that they can still accurately recognize. If an individual can generate an accurate verbal description of an object, it is assumed to have been recognized by them. It is believed that co-existing recognition and naming deficits for man-made objects can occur because the regions mediating these cognitive tasks appear to lie in close proximity in the left posterior temporal region [i.e., left occipital–temporo-parietal (OTP) junction]. Tranel et al. (2006) recently demonstrated that patients with bilateral cerebral lesions sometimes present with both naming and recognition deficits for other object categories (e.g., landmarks, famous faces).1 However, given the atypical language lateralization experienced by our patient, and the location of his tumor in the right anterior TL, he experiences both types of category-specific deficits for unique objects resulting from a single lesion. This suggests that although language is organized in the right hemisphere in this individual, the same neural organization of categorical information has been retained that is usually observed in patients with typical language organization.
Case presentation
The patient is a 32 year-old, left-handed, Caucasian, male with 14 years of education who underwent a right TL resection following the discovery of a right TL tumor presenting with memory complaints, word-finding difficulty, visual changes, and headaches. Although the patient exhibited a left upper visual-field quadrananopsia both pre- and post-operatively, his visual acuity remained average. He did not exhibit any form of visual neglect, and he performed in the average to high average range on measures of visual–spatial processing both pre-and post-operatively.
MRI of the brain revealed a lesion involving the right medial TL, including the hippocampus and parahippocampus, and measuring approximately 2.9 × 2.5 × 3.5 cm in diameter (see Fig. 1). There was no enhancement with MRI contrast administration. The patient subsequently underwent a right temporal craniotomy and resection. He has experienced infrequent seizures since surgery, but is well controlled on lamotrigine, 450 mg daily. His seizures are thought to represent complex partial episodes with secondary generalization. He has no history of experiencing seizures prior to surgery. The patient underwent both pre- and post-operative neuropsychological assessment, MRIs of the brain, an intracarotid amobarbital (Wada) procedure, intraoperative cortical stimulation language mapping, and an 18 month postoperative neuropsychological assessment including specially designed tasks of category-specific recognition and naming ability.
Fig. 1.
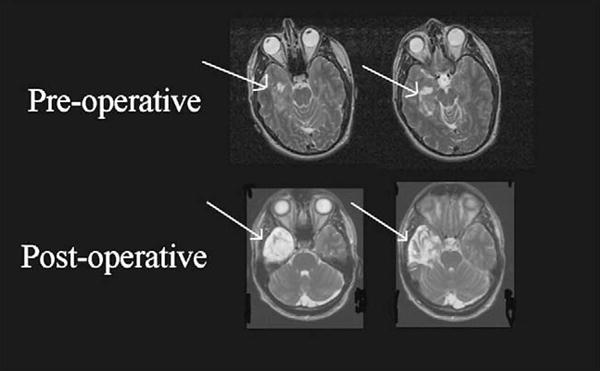
Pre- and post-operative MRI scans showing the patient’s right anterior temporal lobe (TL) tumor and subsequent surgical resection. As with standard clinical convention, the MRI images are flipped in terms of normal left–right orientation (e.g., the left cerebral hemisphere is pictured on the right side of the photograph and the right cerebral hemisphere is pictured on the left).
1.1. Pre-operative neuropsychological assessment and Wada results
The patient completed a neuropsychological assessment in our laboratory approximately 1 month prior to his surgery. At that time, intellectual abilities were substantially above average, with verbal and language-related abilities somewhat stronger than those in the visual–spatial area. His level of academic knowledge was somewhat below his level of general intelligence, but still clearly above average. The patient’s overall neuropsychological assessment was above average, although a small constellation of findings suggested at least mild right-hemispheric dysfunction (see Table 1 for selected neuropsychological scores). These deficits included a relatively poor performance with the left hand on two motor tasks and a relative advantage for verbal/auditory memory versus visual memory. Of note, however, presurgical visual memory ranged from average to high average, while auditory/verbal memory functioning was superior. The patient demonstrated complete left dominance in terms of hand, eye, and foot preference using the Lateral Dominance Examination (Reitan and Davison, 1974). However, he denied having any first-degree relatives who were also left-handed.
Table 1.
Pre- and post-operative neuropsychological assessment scores
| Neurocognitive measures (selected) | Pre-operative assessment | Post-operative assessment |
|---|---|---|
| WAIS-III full-scale IQ | SS = 125 | SS = 103* |
| WAIS-III verbal IQ | SS = 128 | SS = 106* |
| Vocabulary | 13 (scale score) | 10 (scale score)* |
| Similarities | 15 (scale score) | 9 (scale score)* |
| Information | 15 (scale score) | 13 (scale score) |
| WAIS-III performance IQ | SS = 116 | SS = 99* |
| Block design | 13 (scale score) | 11 (scale score) |
| Matrix reasoning | 15 (scale score) | 13 (scale score) |
| Picture arrangement | 10 (scale score) | 10 (scale score) |
| Digit symbol (processing speed) | 16 (scale score) | 8 (scale score)* |
| WAIS-III Working memory index | ||
| Digit span | 17 (scale score) | 12 (scale score)* |
| Arithmetic | 13 (scale score) | 12 (scale score) |
| Letter-number sequencing | N/A | 15 (scale score) |
| WRAT-R reading | SS = 106 | SS = 97 |
| WRAT-R spelling | SS = 108 | SS = 95* |
| WRAT-R arithmetic | SS = 101 | SS = 101 |
| Boston naming test | N/A | Raw Score = 57/60 |
| Aphasia screening test | 0 errors (normal) | 1 error (normal) |
| MAE COWA (total items) | 64 | 58 |
| Semantic fluency (animals total) | 36 | 32 |
| MAE token test | N/A | Raw Score = 44/44 |
| WMS-III auditory memory immediate | SS = 138 | SS = 102* |
| WMS-III auditory memory delayed | SS = 140 | SS = 105* |
| WMS-III visual memory immediate | SS = 112 | SS = 100* |
| WMS-III visual memory delayed | SS = 112 | SS = 110 |
| RAVLT – 5 trial total | 66, T = 66 | 59, T = 60 |
| RAVLT – immediate recall | 14, T = 63 | 12, T = 57 |
| RAVLT – delayed recall | 14, T = 73 | 10, T = 50* |
Note: WAIS-III = 3rd edition of the Wechsler Adult Intelligence Scale; WRAT-R = Revised edition of the Wide Range Achievement Test; MAE = Multilingual Aphasia Examination; COWA = Controlled Oral Word Association; WMS-III = 3rd edition of the Wechsler Memory Scale; RAVLT = Rey Auditory Verbal Learning Test; SS = standard score; T = T-score. Post-operative scores denoted with an asterisk (*) represent a significant decline in performance following surgery. Reliable change indices were utilized when available for individual tests. Such expected change scores have been established for many neuropsychological measures for patients with epilepsy, as this group often undergoes serial assessment (Hermann et al., 1996; Sawrie et al., 1996). No significant improvements were noted following surgery.
The Wada procedure revealed atypical language lateralization, with speech functions lateralized to the right hemisphere only. Wada memory performance was normal following the left injection but mildly impaired following the right injection. This suggested better functioning of the hemisphere to be resected, and indicated that this patient was at significant risk of memory decline with the planned surgical resection involving his dominant (right) TL. Overall, it was felt that this patient’s atypical language lateralization represented a developmental variant of language lateralization rather than the result of a pathological process. Several factors point to this conclusion: (a) there is no evidence that the patient ever experienced any early life trauma or neurological insult involving either cerebral hemisphere; (b) being left-handed, the prevalence rate for atypical speech lateralization is much greater in this individual than it would be for right handed individuals (Knecht et al., 2000); and (c) the patient’s high average to superior baseline cognitive profile would not be expected in someone with pathological reorganization, as such individuals usually experience compromise in one or more cognitive domains. For example, many patients with presumed pathological language reorganization demonstrate decreased visual–spatial processing skills that are presumed to be compromised secondary to language moving into regions intended for other functions (Lidzba et al., 2006). The only finding that could be consistent with a pathological reorganization of language is the mildly compromised Wada memory score that reflects worse memory functioning of the left rather than the right cerebral hemisphere. This could imply that the patient is experiencing some underlying unknown dysfunction of the left hemisphere although it could also be observed in a developmental variant as well.
1.2. Intraoperative language mapping
Intraoperative cortical stimulation language mapping used a standard clinical protocol of naming pictures on a computer screen, with stimuli presented at 3 sec intervals (Ojemann et al., 1989). Once a craniotomy was performed using local anesthetic field block and neuroleptoanalgesia (Silbergeld et al., 1992), the patient was awakened for language mapping. This procedure starts with the determination of an after discharge threshold using electrocorticography. Language mapping is performed with the largest bipolar current that does not evoke after discharges (typically between 1.5 and 10 mA). This is done to avoid propagation of depolarization to nearby cortex, which could lead to false positive results. The patient is shown slides of line drawings of common man-made objects at 3-s intervals, and is trained to name each object as it appears. While the patient performs this task, bipolar current is applied to the cortical surface. Each site is typically stimulated two to three times, though never twice in succession, and at least one slide without stimulation separates each stimulation. A site is considered essential to language function if stimulation produces consistent speech arrest or anomia. In this patient, stimulation at two sites along the posterior aspect of the right superior temporal gyrus produced dysnomia, with these sites located approximately 7 cm back from the anterior temporal tip.
1.3. Surgical parameters
Surgical resection involved the anterior TL, well anterior to the identified speech sites. Intraoperatively, the tumor appeared to involve the parahippocampus and enterorhinal cortex (anterior-inferior TL). It also involved the anteriolateral portion of the hippocampus, although it did not invade the medial border. There was a small layer of grossly normal hippocampus on the mesial border. A gross total resection of the lesion was achieved, which included much of the parahippocampus, enterorhinal cortex, lateral amygdala, and anterior hippocampus (see Fig. 1). Post-operative MRI demonstrated resection of 3.7 cm of inferior temporal gyrus, 3.8 cm of basal temporal cortex, and approximately 2 cm of hippocampus. Final pathology revealed a ganglioglioma and the patient has remained free of recurrence at 1.5 years without adjuvant therapy.
1.4. Post-operative neuropsychological assessment
Standard post-operative neuropsychological assessment was completed approximately 18 months after the patient underwent surgical resection of his tumor. This evaluation revealed significant declines in auditory/verbal memory functioning, with scores dropping from the superior to the average range (see Table 1 for both pre-and post-operative neuropsychological scores). Visual memory functioning also appeared significantly worse post-operatively, with some scores declining from high average to average. IQ scores also declined in a relative fashion, although performance remained average or better. Overall, the patient appeared less adept at processing verbal information (e.g., expressing his thoughts concisely in verbal form, understanding and conveying nuances of word meanings). He experienced significant declines on both the Vocabulary and Similarities subtests from the 3rd edition of the Wechsler Adult Intelligence Scale (Wechsler, 1997). In addition, he missed several items from the WAIS-III Information subscale that required the identification of a specific person or location, which he had answered correctly during his pre-operative evaluation.
The patient did not exhibit a post-operative aphasia, performing in the average or better range on nearly all language measures. This included a normal performance on the Aphasia Screening Test pre- and post-operatively (Halstead and Wepman, 1959), and a high average post-operative performance on a standard visual confrontational naming measure, the Boston Naming Test (Kaplan et al., 1983). He was able to adequately repeat sentences and to perform both simple and complex auditory comprehension tasks that placed demands on understanding complex syntactical structures. Performance on generative verbal fluency tasks, both letter and semantic (animals), was high average pre- and post-operatively, although there did appear to be a slight, but nonsignificant, decline on both following surgery. Of note, these language findings are consistent with our standard dominant anterior TL resection cases, as we typically observe some alteration in naming ability and perhaps verbal fluency (particularly semantic fluency) in the absence of frank aphasia following these surgeries. Classic language disturbance (e.g., positive signs of aphasia in speech, impaired auditory/written comprehension, impaired sentence repetition, abnormal speech fluency) is a rare finding in cases of TL resection, and would only be expected in cases undergoing resections involving the posterior TL (potentially encroaching upon Wernicke’s area).
Visual acuity and visual–spatial functioning appeared average to high average pre- and post-operatively. The patient was able to recognize unfamiliar faces presented from varying angles and with differing degrees of shading. Similarly, he was able to judge the spatial relationships of an array of lines. The patient continued to exhibit a left upper quadrananopsia following his resection and there was no visual neglect. Performance IQ dropped significantly, apparently due to a decline in general processing speed. For example, he exhibited a slight but nonsignificant decline on a block design task, driven by a slow rate of performance. The patient completed all of the same designs as he had pre-operatively, but obtained a lower score as he required additional time to complete them. There was no significant alteration in motor or sensory functioning or executive control processes following surgery.
The patient’s mood appeared worse following surgery, as he presented with some degree of depression. He appeared to be distressed primarily over his decline in neurocognitive ability and its impact upon his functioning. He was participating in counseling at the time of his evaluation to help him adjust to these changes in his functioning.
2. Methods
2.1. Post-surgical category-specific recognition and naming evaluation
The patient was assessed using category-specific stimuli covering two unique (famous faces and famous landmarks) and two nonunique (animals and man-made objects) object categories. All stimuli were obtained from Damasio and colleagues, and have been well validated in a series of studies completed in their own laboratory (Tranel et al., 1997a, 1997b). These stimuli have been widely used to study recognition and naming in these three categories. Visual stimuli included pictures of animals (n = 94), man-made objects (n = 101), famous landmarks (n = 65), and famous faces (n = 155), presented on a laptop computer. The animal domain included several subcategories, including mammals, birds, reptiles, and insects. The man-made object category included a variety of manipulable objects, including common objects used by both genders (e.g., flashlight, shoes, toothbrush). The famous faces included individuals known for their involvement in sports, politics, or entertainment (actors/actresses, musicians). The famous persons subtest includes persons who were famous during the first half of the 20th century (e.g., Charlie Chaplin, Winston Churchill) through the current era (e.g., Oprah Winfrey, George W. Bush, Mel Gibson). No contextual information is included in any of these pictures that might provide clues to their background or reason for notoriety. The famous landmarks stimuli set was recently created by Tranel et al. (2005), and includes photographs of famous geographic landmarks or architectural structures from around the world (e.g., Taj Mahal, the White House, Mount Rushmore, Hoover Dam, Big Ben). Examples of category-specific test stimuli are included in Figs. 2 and 3.
Fig. 2.

Examples of stimuli from the Famous Faces and Famous Landmarks stimuli used in the current study. Examples include John F. Kennedy, Princess Diana, the Space Needle, and the Taj Mahal.
Fig. 3.

Examples of stimuli from the Animals and Man-Made Objects stimuli used in the current study. Examples include a zebra, a penguin, a corkscrew, and a badminton birdie.
For each stimulus, our patient was asked to name the pictured object/person as quickly and accurately as possible. If the initial response was incorrect or consisted of a vague or superordinate response (e.g., “animal” or “movie star”), he was prompted to “be more specific; tell me exactly who [what] you think that [thing] is.” If he still did not come up with the specific name, he was asked to describe the object as completely as possible: “Please describe the object [person] in as much detail as possible, so that I will know that you recognize it [them], even if you cannot recall its [their] specific name.” After each response had been completed, our patient was asked if the object or person was familiar to him (e.g., “Is this someone [something] that you have seen before? Does this person [object] seem familiar to you?”). If he indicated that he was even vaguely familiar with an object or person (even if he did not correctly recognize/identify the object or person), this item was included in later analyses. In contrast, if he denied ever seeing the object or person before, the item was dropped from later analysis for that subject. This patient was familiar with nearly 100% of the animals, man-made objects, and famous landmarks, but was only familiar with 64.5% of the famous faces. Familiarity ratings tend to be lower for famous faces, and sometimes famous landmarks, even for healthy adults, as younger individuals have not always seen some of the famous stimuli (e.g., particularly persons who may have been famous during earlier decades, such as Winston Churchill or Johnny Carson), while older subjects are not always familiar with some of the younger entertainers and sports figures (e.g., Dennis Rodman). In addition, as will be more covered more fully in Section 4, sense of familiarity for famous faces has been shown to be disrupted in patients experiencing right TL lesions (Gainotti, 2007b). Prior to beginning each of these category-specific tests, our patient was given a practice item, with examples of how the stimuli might be described had he not been able to think of its name.
Time limits were not imposed for these category-specific naming and recognition tasks. Responses were tape-recorded and transcribed for later scoring. Scoring methodology followed procedures used by Damasio and colleagues in their prior work in this area (Damasio et al., 1996; Tranel et al., 1997a, 1997b). For each category, we calculated a familiarity score based on our patient’s self-report of whether the object/person was familiar to him or not. This score was calculated by dividing the number of familiar objects by the number of total objects and multiplying by 100. To receive credit for a correct naming response, this patient was required to provide complete object names and both the first and last names of individuals (except ones for whom one name is typical, e.g., “Madonna,” “Cher”). If our patient correctly named an item, he automatically received credit for recognition, which was assumed to be required for accurate naming. For items that were not correctly named, our patient’s transcribed responses were presented to raters who were asked to determine what the stimulus was from the description alone, without having in front of them either the stimulus or its name. If this could be done, the item was scored as a correct recognition; if it could not, then the item was scored as a recognition failure. The recognition score was based only on familiar objects, and was calculated by dividing the total number of objects correctly named or recognized by the total number of familiar objects and multiplying by 100. We next calculated a naming score based only on the recognized objects by adding the total number of objects correctly named and dividing this total by the number of recognized objects and multiplying by 100.
To examine this patient’s level of performance on each of these object categories, his performance was compared to normative data collected by researchers at the University of Iowa College of Medicine. Classification ratings were made for recognition and naming performance for all four of the object categories. Naming accuracy was only rated for items correctly recognized by the patient, making it possible to produce recognition and naming deficits for the same object category. Performance ratings were assigned according to the following classification system: normal (scores no more than 1 SD below the mean of normal subjects), mildly impaired (1–1.5 SD below the mean of normals), moderately impaired (1.5–2 SD below the mean of normals), or severely impaired (>2 SD below the mean of normals) for both naming and recognition scores.
3. Results
The patient exhibited normal recognition and naming performances on the man-made object category. His scores for both of these tasks were average in this object domain. The patient’s percent accuracy scores are included in Table 2 for all object categories.
Table 2.
Percent accuracy scores for both recognition and naming performances on all category-specific tests administered post-operatively
| Percent of objects familiar to the patient (self-report) |
Recognition accuracy (percent recognized of familiar objects) |
Recognition accuracy (percent recognized of total objects) |
Naming accuracy (percent named of recognized objects) |
Naming accuracy (percent named of total objects) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Patient data (%) |
Normative data |
Patient data (%) |
Normative data |
Patient data (%) |
Normative data M (%) (SD) |
Patient data (%) |
Normative data M (%) (SD) |
Patient data (%) |
Normative data |
|
| Man-Made Objects | 101 | 99.0 | N/A | 97.0 | N/A | 96.0 | 96.1 (3.2) | 99.0 | 95.0 (3.1) | 95.0 | N/A |
| Famous Faces | 155 | 64.5 | N/A | 52.0 | N/A | 33.5 | 77.7 (8.0) | 76.9 | 89.3 (4.9) | 25.8 | N/A |
| Famous Landmarks | 65 | 98.5 | N/A | 42.2 | N/A | 41.5 | 62.0 (18.0) | 66.7 | 88.0 (8.0) | 27.7 | N/A |
| Animals | 94 | 100 | N/A | 87.2 | N/A | 87.2 | 93.1 (3.5) | 85.4 | 93.6 (3.0) | 74.5 | N/A |
Note: M = mean; SD = standard deviation.
In contrast, the patient exhibited severe recognition and naming deficits for the famous faces category. As can be seen in Table 2, he performed more than 3 standard deviations (SD) below the mean of the healthy control group on both measures. Specific examples of recognition and naming deficits are included in Table 3.
Table 3.
Examples of naming and recognition errors made post-operatively by the patient on the famous faces subtest
| Famous Faces – test stimuli | Patient responses reflecting recognition errors |
|---|---|
| Bill Gates | “That’s Oliver North. He was involved in the Iran Contra affair… a military man.” |
| George W. Bush | “Richard Nixon… He was the only U.S. President to be impeached.” |
| Susan Sarandon | “Michael Jackson… He is a great pop singer… Now he’s in trouble with the law. |
| Hillary Clinton | “She’s part of the British royal family… a princess or a duchess or something… I don’t recall her name.” |
|
| |
| Famous Faces – test stimuli | Patient responses reflecting naming errors |
|
| |
| Bill Clinton | “He was the last President… I can’t recall his name… He had an affair with an intern. |
| George Burns | “He’s a famous comedian. He lived to be nearly one hundred I think, but he’s dead now. He always smoked big cigars. He also played God in a couple of movies.” |
| Saddam Hussein | “That’s the Iraqi guy… he’s in hiding now because we went after his butt. He’s been leading their country for a long time. The first Bush went after him too.” |
Similarly, the patient exhibited severe recognition and naming deficits for the famous landmarks category. Once again, as with Famous Faces, he performed more than 3 SDs below the mean of the healthy control group on these measures. Specific examples of recognition and naming deficits are included in Table 4.
Table 4.
Examples of naming and recognition errors made post-operatively by the patient on the famous landmarks subtest
| Famous Landmarks – test stimuli | Patient responses reflecting recognition errors |
|---|---|
| Epcot Center | “That’s part of the space center in Florida.” |
| Taj Mahal | “That’s a very beautiful palace that is located somewhere in Russia.” |
| Pentagon | “That’s the White House. That’s where the President lives.” |
|
| |
| Famous Landmarks – test stimuli | Patient responses reflecting naming errors |
|
| |
| The Alamo | “That’s where the Texans were killed during the Mexican War. It was like an old Spanish Church. Davey Crockett was killed there. This fight became the rallying point for the Texans.” |
| Stonehenge | “That’s a site in England… A bunch of large stones in a circle. It probably represented some type of calendar. It is thought to have been used in rituals and I believe it has been associated with the druids.” |
| Leaning Tower of Pisa | “That’s a famous tower in Italy. It is supposedly falling over… I know the name but can’t come up with it.” |
Finally, the patient exhibited what appeared to be generally mild recognition and naming deficits for the category of animals. He performed between 1 and 2 SDs below the mean of the healthy control group for this category. Of note, however, he still correctly identified and named approximately 85% of the stimuli that he recognized from this category. Specific examples of recognition and naming deficits are included in Table 5.
Table 5.
Examples of naming and recognition errors made post-operatively by the patient on the animals subtest
| Animals – test stimuli | Patient responses reflecting recognition errors |
|---|---|
| Fox | “That’s a cat.” (query = can you tell me more about it?) “Sure… You might keep it as a pet. It likes to chase mice.” |
| Bull | “That’s a cow.” (query = can you tell me more about it?) “Just a cow. They produce milk, they have hooves and udders, and live on farms.” |
|
| |
| Animals – test stimuli | Patient responses reflecting naming errors |
|
| |
| Kangaroo | “That’s an animal that hops on its hindlegs… From downunder… It carries its young in its pouch.” |
| Ostrich | “That’s a really large bird… I can’t think of what it’s called. They are known for burying their heads in the sand when frightened. People have been known to ride them at times as well.” |
| Whale | “That’s a large sea mammal. It has a blow hole. Some guy in the Bible got swallowed by one.” |
4. Discussion
This patient with right-hemispheric speech lateralization exhibited both severe recognition and naming deficits for unique objects (famous faces and landmarks) and grossly normal recognition and naming performances for nonunique objects (animals and man-made objects) following resection of an anterior right TL ganglioglioma. Patients with both recognition and naming deficits for famous faces and landmarks have been rare in general, and have only been observed previously in cases with lesions involving both anterior TLs. While recognition deficits have been reported for famous faces following right temporal pole lesions (Tranel et al., 1997a, 1997b; Glosser et al., 2003; Drane et al., 2008), and for landmarks and geographic regions following right TL lesions in general (Landis et al., 1986), these patients have retained the ability to name persons and landmarks/locations that they continued to recognize. In contrast, patients with naming deficits for famous faces and landmarks have typically experienced lesions involving their left anterior TL (Glosser et al., 2003; Damasio et al., 1996; Drane et al., 2008; Tranel, 2006; Yucus and Tranel, 2007), and have continued to demonstrate a normal ability to recognize these objects (i.e., based on their description of the objects/persons). Overall, our patient’s performance is consistent with prior work suggesting that the neuroanatomic substrates for the recognition and naming of unique objects lie in the anterior TL regions. However, his results demonstrate that essential category-specific naming sites can be located in the right anterior TL in cases of atypical language lateralization. In this patient, these particular essential naming sites seem to maintain a pattern of neuroanatomical distribution that is similar to that observed in the left anterior TL of patients with normal (left hemisphere) language lateralization, and there is no evidence of reorganization of recognition processes.
The current pattern of recognition and naming deficits for unique objects with relative sparing of nonunique objects can be anticipated from the Convergence Zone (CZ) model of semantic memory of Damasio and colleagues (Damasio, 1989; Barsalou et al., 2003). This model suggests that intermediary (CZ) sites exist in associational cortex of the brain that function to reactivate stored semantic memories (i.e., memories of concrete entities) that exist in distributed neural networks located in the original sensory and motor areas involved in their perception. The CZ model arose from a series of articles published by Damasio and colleagues using lesion analytic and functional neuroimaging studies to demonstrate that category-specific deficits often occur following a specific pattern of circumscribed lesions (Damasio et al., 1996; Tranel et al., 1997a, 1997b; Grabowski et al., 2001). According to this model, CZ sites primarily located in the nondominant hemisphere are critical for reactivating the stored object percept, while counterparts in the dominant hemisphere (sometimes referred to as mediational zones) function to relate the stored percept to the classic language system in order to attach a verbal label.
Multiple theories have been put forth to explain these apparent segregations in object categories for naming and recognition that exist in the brain (Gainotti, 2006). These have ranged from models based on purported evolutionary significance (Caramazza and Shelton, 1998) to those suggesting that the categories parallel an increasing complexity of analysis performed by the inferior visual pathway as one moves in a posterior to anterior direction in the brain (Bright et al., 2004, 2005). We prefer the latter interpretation, as there is evidence from functional neuroimaging studies that areas of activation produced by a single stimulus can differ significantly depending on the complexity of the visual processing involved in the associated behavioral task (e.g., recognizing a broad category vs a specific item) (Simmons and Barsalou, 2003; Tyler et al., 2004).
While the patient’s ability to name and recognize objects was substantially better for nonunique versus unique objects, he did exhibit at least mild limitations in both of these areas for animals. As can be seen from Table 2, although he exhibited mild deficits relative to healthy control subjects for this category, he still managed to recognize 87% of the animal stimuli and to name approximately 85% of those he recognized. Nevertheless, his response latency was often dramatically slow even for items he correctly named (e.g., sometimes requiring 10–20 sec to respond with the name of a common animal). We have previously reported recognition deficits for animals following right (nondominant) anterior TL dysfunction associated with epileptic focus or subsequent resective surgery, and naming deficits for animals following left (dominant) anterior TL dysfunction (Drane et al., 2004, 2008).
Our patient’s sense of object familiarity was much worse with famous persons than with any other assessed object category. In fact, the patient’s sense of familiarity was almost perfect for the remaining three categories. This finding is consistent with a recent review article by Gainotti that showed decreased familiarity ratings for famous faces across a number of semantic memory and face processing studies (Gainotti, 2007b).
One limitation of the current study is a lack of pre-operative category-specific recognition and naming data. Without such data we cannot definitively attribute post-operative deficits to the impact of surgery versus the underlying disease process. Nevertheless, given that the patient did not exhibit any language deficits pre-operatively, and that his verbal processing appeared worse post-operatively, one has to suspect that these category-specific deficits are related to the surgical intervention. As noted, the patient appeared less adept at expressing his thoughts concisely in verbal form and understanding and conveying nuances of word meanings. He also exhibited significant declines on the Vocabulary, Similarities, and Information subtests from the WAIS-III (which included not being able to think of the names of historical individuals on items that he had correctly answered pre-operatively).
While we do not see it as a limitation, our methodological approach for studying object recognition and naming differs from that of researchers coming from the perspective of cognitive psychology. Our assessment approach is rooted firmly in the lesion analysis paradigm, which tends to start by exploring the behavioral phenomena and its neural underpinnings, and it is driven by clinical concerns arising in the context of neurosurgery (e.g., defining and preventing deficits from surgery in these areas). Therefore, we look at naming response speed by having the patient initially name the pictured object, emphasizing that each item is to be named as quickly and accurately as possible. This allows us to examine post-surgical declines in response rate, which our work is suggesting can negatively impact other essential aspects of verbal processing. In contrast, studies arising from the cognitive psychology framework tend to start with theoretical models of object processing (Bruce and Young, 1986), and seek to verify the various hypotheses that can be generated from these models (Gainotti, 2007a). These latter approaches have frequently defined object recognition differently than the lesion analysis approach, and traditionally start by assessing object familiarity, then recognition, and finally naming ability, to be consistent with the stages of processing proposed by models from this paradigm. Using this approach, one cannot reliably assess naming response rate, as the subject is required to complete intervening tasks prior to ever naming the object. We remain just as interested in these earlier stages of processing (e.g., sense of object familiarity), and believe that we accurately capture all of the same information (and more) with our current paradigm.
Our method of defining recognition also differs from the approach of some of the cognitive psychology/neuroscience paradigms for studying object processing. Specifically, while we require the patient to either name the object or to provide an accurate description of the object in order for it to be counted as a correct recognition, some prior studies have instead assumed correct recognition if the subject can provide a general categorical description of an object (e.g., state the occupation of an individual on a famous faces test) or in the case of unique objects, pick the object that is thought to be famous from a selection of two or more foils. We believe that both of these latter approaches are prone to error and may not adequately demonstrate that recognition has occurred. For example, our patient was sometimes able to accurately provide a general descriptor for an object (e.g., “That’s probably an athlete” when presented with a picture of Muhammed Ali), although he denied having any sense that the object was familiar, and went on to provide an erroneous verbal description (e.g., “He probably plays football or something”). When his responses were later queried, the patient often indicated that he came up with a general descriptor label based on the physical qualities of the object even in the absence of a sense of familiarity (e.g., “big, bulky guys are probably some type of athlete rather than a musician, politician, or whatever.”). Defining recognition on the basis of choosing from a group of foils may also be in error, as this decision could be performed on the basis of an intact sense of familiarity alone. Therefore, those researchers counting these items as correct recognitions, and subsequently including these items in their calculation of a naming score, are most likely mislabeling a recognition deficit as a naming error. These potentially problematic methods for defining recognition have typically been used in the studies that have reported naming deficits following right anterior TL dysfunction (a finding that does not occur using our methodology in individuals with typical language lateralization).
The functional significance of category-specific naming and recognition deficits remains unclear. Others have suggested that recognition deficits lead to difficulty in general social functioning (Glosser et al., 2003). For example, individuals may not even recognize their own family members or be able to learn new faces in a social or work environment. We have also speculated that category-specific naming deficits may contribute to difficulty with both verbal processing and expression (Drane et al., 2004, 2008), particularly for those whose response latencies have become remarkably slow (e.g., going from being able to come up with the name of an object in less than 2 sec to requiring greater than 10 sec for the same response). In turn, such naming deficits may make it more difficult to encode some types of verbal information and to engage in certain verbal reasoning tasks. For example, one might expect someone undergoing an anterior dominant TL resection to have greater difficulty putting names with faces and locations. These concerns are particularly relevant in surgical cases where options are available regarding the necessity or extent of resection (e.g., epilepsy surgical interventions).
The pattern of identified naming sites in this patient determined through intraoperative cortical stimulation language mapping appeared consistent with the maps of patients whose left cerebral hemisphere is dominant for language. In a large study of patients undergoing intraoperative language assessment (Ojemann et al., 1989), it was previously demonstrated that the most common pattern of language localization involved two essential naming regions (often as small as 1 cm2 each), with one in the frontal lobe and a second in the temporo-parietal area. In addition, despite a great deal of variability across patients, over 1/3 of patients exhibited essential naming sites in this same region of the posterior superior temporal gyrus. The current results suggest it is possible for atypical (right hemisphere) language development to parallel the normal distribution of essential naming sites that is seen in patients with left hemisphere language dominance. Thus, it is possible that atypical language development might occur as a normal but rare variant of standard language development rather than reflecting a pathological process. This is further suggested by the presence of the patient’s tumor in the right rather than the left hemisphere, as one would not expect a right-sided insult to lead to atypical language development. These intraoperative language findings also validate the Wada evaluation, as they demonstrate that essential naming sites can be found in individuals shown to have exclusively right hemisphere language by this exam.
Finally, given that various categories of items may be represented by different regions within the TLs, it is possible that including a broader sampling of stimuli during cortical stimulation language mapping would provide a broader map of language function (Hamberger et al., 2005; Ojemann et al., 1996). The stimuli included in most cortical stimulation language mapping procedures tend to include only man-made objects. As we noted in the introduction, both recognition and naming of man-made objects appear to be mediated by neuroanatomical sites in the posterior TL rather than the anterior region of this structure. Therefore, we may not be adequately assessing the region that is more commonly resected during surgery to control TL seizure onset. Whether a different, or complementary, set of stimuli will prove more useful for preventing recognition and naming deficits for the various categories of objects examined in the current study is a topic of future research. It is unknown whether focal sites can be identified for face and landmark objects in the same spatial manner as seen for conventional mapping with man-made objects. Future research should explore the use of a broader array of stimulus categories during cortical stimulation language mapping to determine if different language maps are uncovered for different categories of stimuli. Whether sparing of those structures is either practical or desirable in the presence of anterior TL pathology will also require investigation and likely remain an individualized decision depending on the patient, surgeon, and anticipated pathology.
Acknowledgments
This research was supported in part by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institute of Health (NIH) (Grant Number: K23 NSO49100-01). This study was approved by the Institutional Review Board (application #05-5738-G 01) of the University of Washington Medical School, where all data were collected. We would like to thank Drs. David W. Loring and Gregory P. Lee for input regarding language lateralization issues with this patient.
Footnotes
It should be noted that some studies report naming deficits related to right hemisphere neurological insults. However, these studies typically use a methodology that counts all naming failures as errors rather than our practice of only examining naming performance for those objects that the patient was able to recognize. We believe that such paradigms misattribute what is actually a problem with recognition or item familiarity to naming impairment.
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- Barsalou LW, Simmons WK, Barbey AK, Wilson CD. Grounding conceptual knowledge in modality-specific systems. Trends in Cognitive Sciences. 2003;7:84–91. doi: 10.1016/s1364-6613(02)00029-3. [DOI] [PubMed] [Google Scholar]
- Bright P, Moss H, Tyler LK. Unitary vs multiple semantics: PET studies of word and picture processing. Brain and Language. 2004;89:417–432. doi: 10.1016/j.bandl.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Bright P, Moss HE, Stamatakis EA, Tyler LK. The anatomy of object processing: the role of anteromedial temporal cortex. The Quarterly Journal of Experimental Psychology. 2005;58B:361–377. doi: 10.1080/02724990544000013. [DOI] [PubMed] [Google Scholar]
- Bruce V, Young AW. Understanding face recognition. British Journal of Psychology. 1986;77:305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Shelton JR. Domain-specific knowledge systems in the brain: the animate–inanimate distinction. Journal of Cognitive Neuroscience. 1998;10:1–34. doi: 10.1162/089892998563752. [DOI] [PubMed] [Google Scholar]
- Chan AS, Salmon DP, De La Pena JH. Abnormal semantic network for “Animals” but not “Tools” in patients with Alzheimer’s disease. Cortex. 2001;37:197–217. doi: 10.1016/s0010-9452(08)70568-9. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33:25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio A. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Drane DL, Ojemann GA, Tranel D, Ojemann JG, Miller JW. Category-specific naming and recognition deficits in patients with temporal lobe epilepsy. Journal of the International Neuropsychological Society. 2004;10:202. [Google Scholar]
- Drane D, Ojemann G, Aylward E, Ojemann J, Johnson L, Silbergeld D, et al. Category-specific naming and recognition deficits in temporal lobe epilepsy. Neuropsychologia. 2008;46:1242–1255. doi: 10.1016/j.neuropsychologia.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G. Anatomical functional and cognitive determinants of semantic memory disorders. Neuroscience & Biobehavioral Reviews. 2006;30:577–594. doi: 10.1016/j.neubiorev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Gainotti G. Different patterns of famous people recognition disorders in patients with right and left anterior temporal lesions: a systematic review. Neuropsychologia. 2007a;45:1591–1607. doi: 10.1016/j.neuropsychologia.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Gainotti G. Face familiarity feelings, the right temporal lobe and the possible underlying neural mechanisms. Brain Research Reviews. 2007b;56:214–235. doi: 10.1016/j.brainresrev.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Glosser G, Salvucci AE, Chiaravalloti ND. Naming and recognizing famous faces in temporal lobe epilepsy. Neurology. 2003;61:81–86. doi: 10.1212/01.wnl.0000073621.18013.e1. [DOI] [PubMed] [Google Scholar]
- Grabowski T, Damasio H, Tranel D, Ponto LLB, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entries. Human Brain Mapping. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead W, Wepman J. The Halstead–Wepman aphasia screening test. Journal of Speech Language and Hearing Research. 1959;14:9–15. doi: 10.1044/jshd.1401.09. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, Mckhann GM, Perrine K, Goodman RR. Brain stimulation reveals critical auditory naming cortex. Brain. 2005;128:2742–2749. doi: 10.1093/brain/awh621. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Schoenfeld J, Peterson J, Leveroni C, Wyler AR. Empirical techniques for determining the reliability, magnitude, and pattern of neuropsychological change after epilepsy surgery. Epilepsia. 1996;37:942–950. doi: 10.1111/j.1528-1157.1996.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Koenig P, Smith EE, Glosser G, De Vita C, Peachie M, McMillan C, et al. The neural basis for novel semantic categorization. Neuroimage. 2005;24:369–383. doi: 10.1016/j.neuroimage.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Landis T, Cummings JL, Benson DF, Palmer EP. Loss of topographic familiarity: an environmental agnosia. Archives of Neurology. 1986;43:132–136. doi: 10.1001/archneur.1986.00520020026011. [DOI] [PubMed] [Google Scholar]
- Laws KR, Sartori G. Category deficits and paradoxical dissociations in Alzheimer’s disease and herpes simplex encephalitis. Journal of Cognitive Neuroscience. 2005;17:1453–1459. doi: 10.1162/0898929054985428. [DOI] [PubMed] [Google Scholar]
- Lidzba K, Staudt M, Wilke M, Grodd W, Krageloh-Mann I. Lesion induced right-hemispheric language organization of nonverbal functions. Neuroreport. 2006;17:929–933. doi: 10.1097/01.wnr.0000221841.12632.d6. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. Journal of Neurosurgery. 1989;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Ojemann J, Ojemann G, Lettich E. Cortical stimulation mapping of category-specific naming. Neuroimage. 1996;3:S451. [Google Scholar]
- Reitan RM, Davison LA, editors. Clinical Neuropsychology: Current Status and Applications. Washington, DC: V.H. Winston & Sons; 1974. [Google Scholar]
- Sawrie SM, Chelune GJ, Naugles RI, Luders HO. Empirical methods for assessing meaningful neuropsychological change following epilepsy surgery. Journal of the International Neuropsychological Society. 1996;2:556–564. doi: 10.1017/s1355617700001739. [DOI] [PubMed] [Google Scholar]
- Silbergeld DL, Mueller WM, Colley PS, Ojemann GA, Lettich E. Use of propofol (diprivan) for awake craniotomies: technical note. Surgical Neurology. 1992;38:271–272. doi: 10.1016/0090-3019(92)90038-o. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Barsalou LW. The similarity-in-topography principle: reconciling theories of conceptual deficits. Cognitive Neuropsychology. 2003;20:451–486. doi: 10.1080/02643290342000032. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Thompson JC, Neary D. Knowledge of famous faces and names in semantic dementia. Brain. 2004;127:860–872. doi: 10.1093/brain/awh099. [DOI] [PubMed] [Google Scholar]
- Tranel D. Impaired naming of unique landmarks is associated with left temporal polar damage. Neuropsychology. 2006;20:1–10. doi: 10.1037/0894-4105.20.1.1. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997a;35:1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Tranel D, Logan CG, Frank RJ, Damasio AR. Explaining category-related effects in the retrieval of conceptual and lexical knowledge for concrete entities: operationalization and analysis of factors. Neuropsychologia. 1997b;35:1329–1339. doi: 10.1016/s0028-3932(97)00086-9. [DOI] [PubMed] [Google Scholar]
- Tranel D, Enekwechi N, Manzel K. A test for measuring recognition and naming of landmarks. Journal of Clinical and Experimental Neuropsychology. 2005;27:102–126. doi: 10.1080/138033990513663. [DOI] [PubMed] [Google Scholar]
- Tranel D, Feinstein JS, Manzel KW. Further lesion evidence for the neural basis for retrieving conceptual knowledge for concrete entities. Society for Neuroscience Abstracts. 2006;608:8. [Google Scholar]
- Tyler L, Stamatakis E, Bright P, Acres K, Abdallah S, Rodd J, et al. Processing objects at different levels of specificity. Journal of Cognitive Neuroscience. 2004;16:351–362. doi: 10.1162/089892904322926692. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;197:829–854. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale: Administration and Scoring Manual. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Yucus C, Tranel D. Preserved proper naming following left anterior temporal lobectomy is associated with early age of seizure onset. Epilepsia. 2007;48:2241–2252. doi: 10.1111/j.1528-1167.2007.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


