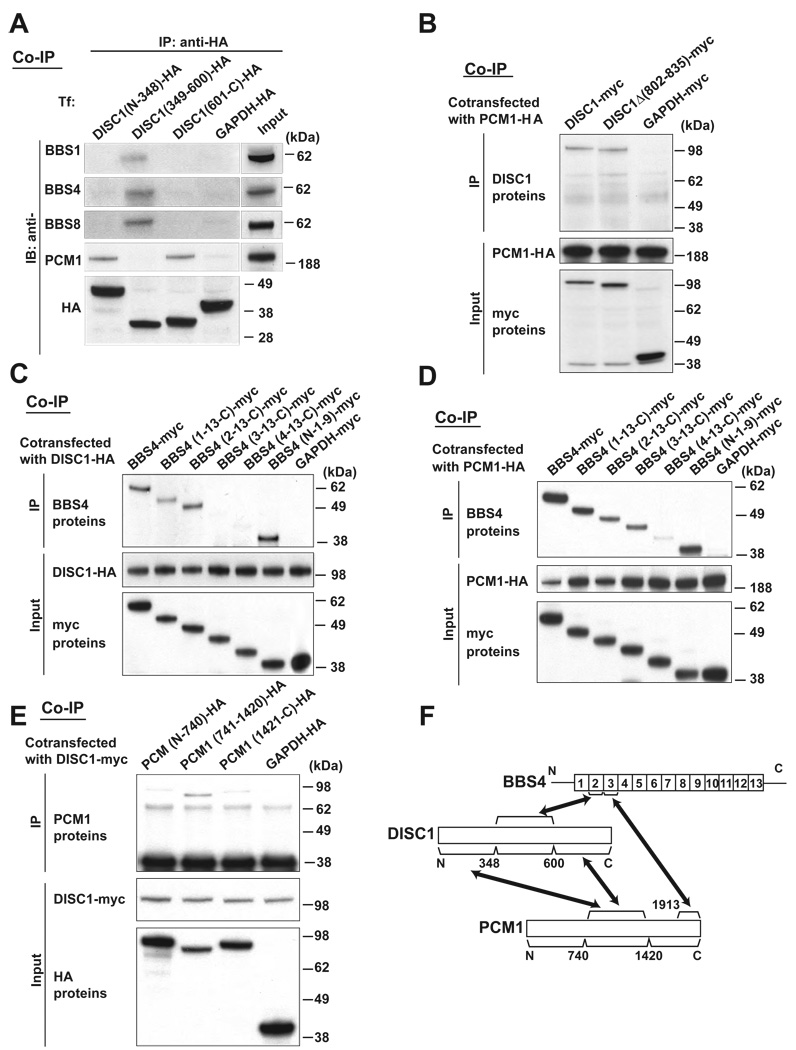
Figure 2. PCM1, DISC1, and BBS4 interact with each other through distinct binding domains.
(A) The middle portion of DISC1 (amino acids 349–600) is crucial for DISC1-BBS4 protein interaction. The N-terminal portion (amino acids 1–348) and the C-terminal portion (amino acids 601–854) of DISC1 are important for the DISC1-PCM1 binding. HA-tagged three DISC1 protein fragments [DISC1 (N-348), DISC1 (349–600), and DISC1 (601-C)] were expressed in HEK293 cells for co-immunoprecipitation with an anti-HA antibody. The middle portion of DISC1, but not the N- nor C-terminal DISC1, binds to each of BBS1, 4, and 8, whereas the N- and C-terminal DISC1 bind to PCM1 (upper panels). The inputs of each protein are shown at the right and bottom panels. IB indicates antibodies used for Western blotting.
(B) The C-terminus domain of DISC1 for interaction with PCM1 is distinct from the NDEL1 binding domain of DISC1. Deletion of DISC1-NDEL1 binding region [DISC1Δ(802–835)] had no effect on the interaction of DISC1 with PCM1. The inputs are shown in the middle and bottom panels.
(C) The second TPR motif of BBS4 is crucial for the BBS4-DISC1 interaction. A series of myc-tagged BBS4 truncation mutants were co-expressed with HA-tagged DISC1 in HEK293 cells for co-immunoprecipitation with an anti-HA antibody. Deletion of the N-terminal region in [BBS4 (1-13-C)] and further deletion of the first TRP motif [BBS4 (2-13-C)] does not affect the BBS4-DISC1 interaction. By contrast, BBS4 mutants with further deletion of the second TRP motif [BBS4 (3-13-C) and BBS4 (4-13-C)] did not bind with DISC1. BBS4 lacking the C-terminal region [BBS4 (N-1-9)] bind to DISC1. The inputs are also shown (middle and bottom panels).
(D) The third TPR motif of BBS4 is important for the BBS4-PCM1 interaction. Deletion of the third TPR motif [BBS4 (4-13-C)] dramatically weakened the interaction of myc-tagged BBS4 with HA-tagged PCM1. An anti-HA antibody was used for co-immunoprecipitation. The inputs are shown in the middle and bottom panels.
(E) The middle portion of PCM1 is important for DISC1-PCM1 interaction. Myc-tagged DISC1 was co-expressed with HA-tagged PCM1 protein fragments in HEK293 cells for co-immunoprecipitation with an anti-myc antibody. PCM1 (741–1420) has stronger binding affinity to DISC1 than PCM1 (N-740) and PCM1 (1421-C). The inputs of each protein are also shown in middle and bottom panels.
(F) Schematic of DISC1, BBS4, and PCM1 interaction shows that these proteins may interact with each other through distinct binding domains.