Abstract
Infection by measles virus (MV) is a major cause of human morbidity and mortality worldwide. In 2001, the WHO, UNICEF and their partners launched the Measles Initiative, the goals of which are to interrupt the transmission of MV in large geographic areas by increasing vaccination coverage and to assess the feasibility of eradicating MV worldwide. An estimated 74% reduction in mortality resulting from measles was achieved between 2000 and 2007, equivalent to a reduction of approximately 200,000 deaths annually. Despite this progress in the control of measles, the highest number of measles cases in more than a decade was observed in 2008 in several European countries and the US, and the virus was again declared endemic in the UK. In the light of this resurgence in the UK and the limitations associated with the current live-attenuated vaccine, this review discusses the means by which safe and effective measles antivirals could augment vaccination and strengthen global efforts to control measles. Important aspects of treatment are the potential to prevent infection effectively after exposure to MV, the improvement of case management, the amelioration of complications that frequently follow MV infection and the influence of antivirals on a potential strategy for global measles eradication.
Keywords: Antiviral, drug, eradication, measles, vaccine, virus
Introduction
Measles virus (MV) is a member of the Morbillivirus genus in the Paramyxovirus family. MV and other paramyxoviruses, such as mumps virus, respiratory syncytial virus, human parainfluenzaviruses and recently emerged zoonotic hendra and nipah viruses, constitute major pathogens for humans and animals [1]. All of these viruses are highly communicable airborne pathogens that spread via the respiratory route. Furthermore, MV is one of the most infectious viruses identified, with a basic reproduction number (R0) of 12 to 18 [2-4], meaning that a single infection will cause 12 to 18 secondary cases in a fully susceptible population in the absence of intervention. High infectivity combined with the induction of long-lasting immunity protecting individuals against re-infection means that a population size of approximately 250,000 individuals or greater is required to ensure sufficient births of susceptible individuals to sustain continued MV transmission [5,6]. Because the virus has no non-human reservoir [2,3], MV can have emerged only after human populations reached this size approximately 5000 years ago [3]. As such, measles is a comparatively young human disease.
MV as an infectious agent
Paramyxovirus particles possess a lipid envelope derived from the host-cell plasma membrane and a nonsegmented, single-strand RNA genome of negative polarity [7]. Paramyxovirus virions are of pleiomorphic shape with an average diameter of 150 to 300 nm. Inserted into the MV envelope are glycoprotein spikes (Figure 1), consisting of the attachment (H) and fusion (F) proteins, which mediate receptor binding, subsequent membrane fusion of the virus, and cellular entry. A helical nucleocapsid core consisting of the RNA genome and the nucleocapsid (N), phospho- (P) and large (L) proteins are tethered to the envelope by the matrix (M) protein (Figure 1B) [1,3].
Figure 1. Measles virus, a member of the Paramyxovirus family.
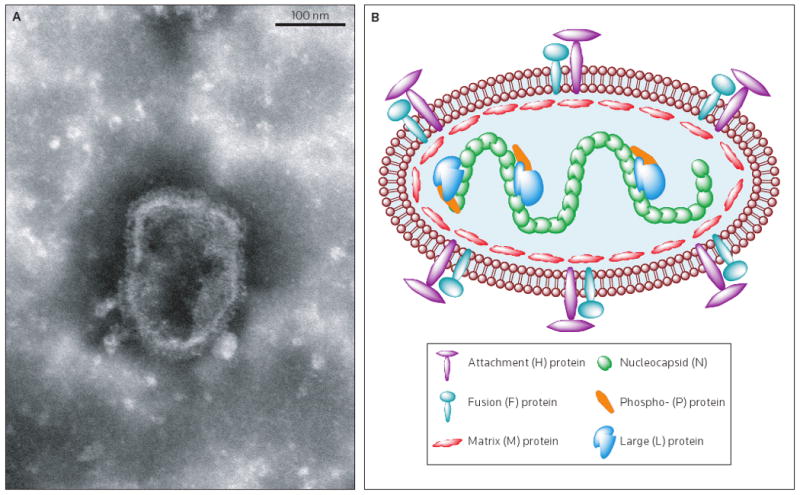
(A) Electron micrograph of purified MV particles at a magnification of 100,000×. Virions are pleiomorphic with an approximate diameter of 150 to 300 nm. Glycoproteins embedded in the viral envelope are detectable (Image was taken by Brindley MA, Wang JJ and Plemper RK). (B) Schematic representation of an MV particle showing the six structural viral proteins. Not shown are non-structural C and V proteins that are encoded in the P gene and accessed through alternative ribosome initiation and pseudotemplated RNA editing, respectively. Only a small number of envelope glycoprotein complexes are shown for clarity.
Signaling lymphocyte activation molecule (SLAM) is a cellular receptor for all characterized MV strains [8,9]. Some laboratory-adapted and vaccine strains of MV can also use the regulator of complement activation (CD46) for efficient cellular entry [10-12]. Receptor binding triggers pH-independent fusion of the viral envelope with the target plasma membrane, followed by the release of the incoming nucleocapsid core into the target cell. Transcription of viral mRNAs and genome replication – the latter via a positive-sense, full-length antigenome – then occurs in the cytosol, mediated by the virus-encoded, RNA-dependent RNA-polymerase (RdRp) complex [13].
Similar to other members of myxovirus families, MV RdRp consists of the viral P and L proteins in addition to the N:RNA template [1]. The non-coding 5′ and 3′ termini of the viral genome contain cis-acting promoter and encapsidation signals that initiate the synthesis of mRNAs or antigenome, or genome replication [14]. The final assembly of newly synthesized nucleocapsid cores and glycoprotein complexes, enveloping, and budding of progeny viral particles occur at the plasma membrane of infected cells [1].
Multiple steps in the paramyxovirus life cycle are unique to the virus compared with the host cells, and thus constitute attractive targets for pathogen-directed antiviral therapies. Promising pharmacological approaches may include viral entry inhibitors that prevent receptor binding, envelope protein refolding or membrane merger required for infection, and inhibitors of RdRp that suppress viral mRNA synthesis and genome replication. Such viral mechanisms can potentially be targeted safely because human cells lack structural or functional homologs of these viral proteins.
MV pathogenesis and diseases potentially associated with MV infection
MV is transmitted by respiratory secretions from infected individuals, either person-to-person via larger respiratory droplets or airborne via aerosols. Initially after infection of a susceptible host, the replication of the virus is supported by dendritic cells in the respiratory tract, lymphocytes and regional lymphatic tissues [15]. This phase of localized replication is followed by primary (2 to 3 days after invasion) and secondary (5 to 7 days after invasion) viremia, with viral spread to multiple organs including the kidney, liver, gut and skin [2,3,16] and predominantly to SLAM-positive lymphocytes and dendritic cells [17]. After an average incubation period of 10 to 12 days, clinical features of acute measles include a prodromal fever, a respiratory infection with rhinitis and severe cough, coryza, conjunctivitis, and pathognomonic enanthem [18]. The maculopapular rash that characterizes measles then appears approximately 2 to 4 days after the prodrome [3,19]. Shedding of the virus occurs from the nasopharynx from the onset of the prodrome until 3 to 4 days after the beginning of the rash [18].
Complications of severe measles infection can include acute demyelinating encephalomyelitis (ADEM) [20] and measles inclusion body encephalitis (MIBE) [19], which manifest soon (weeks to months) after infection. A lethal late complication, subacute sclerosing panencephalitis (SSPE), can present years after the primary infection [3,19,21] and is largely untreatable with currently available therapeutics (see discussion in the Current and experimental drugs for the management of measles section). Worldwide, there were approximately 200,000 measles-related deaths in 2007, rendering the virus a major cause of human morbidity and mortality [22]. A prolonged state of immunosuppression of several months that follows acute cases of measles frequently predisposes patients to bacterial otitis media and bronchopneumonia [19]. Most deaths associated with measles are attributable to secondary viral, bacterial or parasitic infections that occur in this immunocompromised state [3,21].
In addition to direct complications and secondary infections associated with MV infection, a potential MV-related etiology has been discussed for a variety of persistent human diseases including rheumatoid arthritis [23] and multiple sclerosis [24-26]. A possible contribution of MV to lung cancer and Hodgkin's lymphoma has similarly been investigated [27-29]. However, a clear causal relationship between MV infection, and these and several other sequelae cannot be established based on the currently available data. That is, the link between the virus and numerous patient conditions is not supportable by the scientific evidence (for a comprehensive review, see reference [21]).
Measles vaccination and global control efforts
MV meets several prerequisites essential for possible global eradication, including an absence of a non-human reservoir, availability of accurate diagnostic tests and the existence of an effective vaccine [30]. In 2001, the WHO, UNICEF and their partners launched the Measles Initiative, the goals of which are to interrupt the transmission of MV in large geographic areas by increasing vaccination coverage and to assess the feasibility of the worldwide eradication of MV [31,32]. Since this program was established, the number of fatalities caused by worldwide measles was approximately 200,000 in 2007, an estimated 74% decrease compared with 2000 [22], and the virus is no longer considered endemic in the Americas [33].
Despite the successful application of the current live-attenuated measles vaccine [34], several factors contribute to the ongoing morbidity and mortality associated with MV worldwide. First, a trend of increasing measles case numbers has appeared in several industrialized countries, mostly as a consequence of elective exemption from vaccination because of personal or parental philosophical or religious beliefs [35,36]. As a result of its high infectivity, measles is one of the first diseases to reappear when vaccination rates decline [35]. Although in the European region (as defined by the WHO) the incidence of measles was reduced from 8223 cases in 2006 to 3909 in 2007 [36], the highest numbers of cases in more than a decade were observed in several European countries in 2008 [35,37,38]. The UK alone reported 1217 cases from January to November 2008 [39], placing it together with Romania, Germany, Switzerland and Italy as one of the European countries with the most cases of measles [40]. In June 2008, the virus was again declared endemic in the UK [39,41], 14 years after it had been eliminated, and it appears unlikely that the goal of elimination in Europe by 2010 can be achieved [40]. In the UK in particular, parental concerns about vaccination safety were heightened by a report that associated the trivalent measles-mumps-rubella (MMR) vaccine with the onset of autism and intestinal disease [42]; a claim that has since been demonstrated to be unsubstantiated [43-45]. When accompanied by a decline in public awareness of the disease, a scenario of waning immunity based primarily on philosophical beliefs cannot be excluded for the US. The number of cases of measles in the US also reached a 10-year high in 2008, mainly as a result of greater transmission after importation of the virus [35].
Second, due to the high communicability of the virus, a susceptibility of 5 to 6% of an otherwise highly vaccinated population is sufficient to sustain periodic outbreaks [4,46]. A ‘herd immunity’ of greater than 95% [47] is thus required for complete suppression of the virus, which cannot be achieved with a single dose of the vaccine [46,48]. While a second vaccination is routinely administered in developed countries [49,50], there are greater logistical obstacles to repeated vaccination in the developing world. In particular, the current live-attenuated vaccine requires an uninterrupted cold-chain [18], sterile materials and professional healthcare workers for administration [51].
Third, the efficiency of vaccination in infants younger than 12 months of age is compromised by the immaturity of their immune systems and interference from transplacentally acquired maternal antibodies [51-53]. Because maternal antibody titers vary, the immunity of infants to MV is frequently lost at 4 to 9 months of age, creating a window of susceptibility for infection by MV prior to vaccination [51].
Furthermore, concerns have been raised that efforts to eradicate MV might be compromised in the long term by waning protection of the adult population because immunity against the attenuated vaccine strain is less durable than that acquired naturally [54-56]. While currently available data indicate that this might not constitute a major obstacle to the control of MV [30,57,58], the concern of waning immunity could undermine public confidence in vaccination against MV.
Potential role of antiviral therapeutics in the management and control of MV
A novel platform combining prophylactic (vaccination) and therapeutic (antiviral) approaches could overcome both currently encountered and possible future obstacles to the control of MV. However, such an approach would require the identification of antivirals that are safe and effective against MV.
Antiviral drugs can facilitate the rapid control of local viral outbreaks in industrialized and developing countries through post-exposure prophylaxis of the immediate, non-immunized contacts of identified cases in the family and community settings, such as in childcare centers and schools. The long incubation period of MV prior to the onset of viremia offers a large potential window for antiviral treatment. In particular, rapid pre-emptive antiviral treatment could suppress the development of disease entirely in naïve individuals or reduce the severity and longevity of clinical symptoms if naïve individuals are infected before they are vaccinated (either because the vaccination is refused or unavailable). Conversely, long incubation periods may compromise therapeutic potential because clinical disease follows the peak of virus replication. However, the reduction of MV-induced respiratory distress by treatment with ribavirin (see discussion in the Current and experimental drugs for the management of measles section) [59-61], provides some support for the hypothesis that antivirals administered at the stage of clinical disease may improve the management of severe cases. It is possible that such treatment might reduce the disease burden and ameliorate complications and, conceivably, antivirals might also open novel therapeutic options for the treatment of late measles sequelae such as SSPE.
The use of antivirals against MV could, furthermore, contribute to removing the ‘window of susceptibility’ that is present when maternal antibody titers decrease in infants, by post-exposure prophylaxis prior to vaccination. Preventing this type of transmission would be of special importance in areas of the developing world where the virus is endemic, and in those industrialized countries where declining herd immunity has resulted in the return of endemic MV transmission.
Several logistical advantages render small-molecule antivirals particularly suitable for rapid application in the developing world: large scale production strategies are generally well-established and typically highly cost-effective; small molecules can frequently be optimized to achieve high shelf-stability at ambient temperatures (unlike the MMR vaccine that requires uninterrupted cold-chains); and compounds can be formulated for aerosolized or oral bioavailability, allowing rapid mass administration. The field strategy for the advanced stages of global MV eradication proposed by the Pan American Health Organization predicts the need for ‘mop-up’ campaigns to target susceptible children in difficult-to-reach sites of viral outbreaks [62]. Safe and effective antivirals could constitute a desirable additional component of such campaigns because these drugs could provide immediate control of local outbreaks before the trained personnel and sterile materials required for vaccination are available on site.
The HIV/AIDS pandemic is reducing the effectiveness of vaccination for MV; increased rates of failure of both primary and secondary measles vaccination [63] and prolonged shedding of MV have been reported in children infected with HIV [64]. It has been suggested that the high mortality rate among HIV-positive children in the developing world might preclude the formation of a sufficiently large group of MV-susceptible individuals to sustain transmission of MV [2]. However, improved access to antiretroviral therapy may lead to an increased need for effective measles therapy.
The Global Polio Eradication Initiative demonstrated that in the endgame of viral eradication, outbreaks in countries with remaining endemic transmission – in the case of poliovirus, Nigeria, India, Afghanistan and Pakistan [65] – can result in viral spread across several continents [66]. It is pertinent to consider the different routes of transmission and basic reproduction numbers of MV and poliovirus: MV undergoes airborne transmission and has an R0 value of 12 to 18 whereas the transmission of poliovirus is fecal-oral and the R0 value is 5 to 7 [67]. Therefore, sporadic outbreaks of MV comparable to those with poliovirus at a very late stage of eradication could lead to even more rapid viral spread across large geographic areas. The relevance of this scenario will likely be increased once the virus is considered to be nearly eliminated. At this time, the resources in industrialized countries will be sufficient to continue vaccination programs [2,30,51]. This must be offset, however, against the possibility of a rapid erosion of public acceptance and global political will to maintain global vaccination against an ‘eradicated’ pathogen. Thus, a back-up antiviral prophylaxis strategy to immediately curb local outbreaks through stockpiled, shelf-stable antivirals until mop-up vaccination responses can be implemented in the area would be desirable and likely boost public confidence.
Current and experimental drugs for the management of measles
Currently, no therapeutics for the treatment of measles are available. Ribavirin (which is approved for the treatment of some paramyxovirus infections) and IFNα therapies have been tested clinically against MV, mostly for the treatment of patients presenting with SSPE. Although some studies noted a beneficial effect of IFNα [68,69], the majority of reports documented either a long-term relapse [70-72] or lack of efficacy [73-76]. High-dose ribavirin treatment, either alone or in combination with IFNα, appeared to be more efficacious against MV than IFNα alone [59-61,70,77,78]. Although some studies noted a gradual progression of measles despite therapy [72,74] or a lack of efficacy [73], these reports nevertheless suggest that antivirals might ameliorate the complications of measles even when administered after the onset of clinical symptoms. However, severe side effects, most notably hemolytic anemia, have been attributed to ribavirin when used in combination with pegylated IFNα for the treatment of viral hepatitis C [79,80]. Treatment with high-dose vitamin A has been associated with some reduction in the morbidity and mortality of measles [81], but this effect is most pronounced in children younger than 2 years of age [82,83]. For post-exposure prophylaxis, the administration of high-titer MV-specific immune globulin (Ig) within 6 days of exposure can prevent or modify disease [84]. Such treatment is recommended for temporary protection of the immunocompromised and infants younger than 1 year of age [18]. However, Ig therapy is comparatively expensive, requires sterile materials and an uninterrupted cold-chain, and is overall not recommended or feasible across an entire population for the control of large measles outbreaks.
Considering these mixed reports of efficacy and the additional limitations associated with Ig and ribavirin therapies, the development of novel, safe and efficacious inhibitors of MV is required for a combined prophylactic and therapeutic anti-measles platform. Other desired features of a measles antiviral are cost-effective mass production, shelf-stability and the potential for oral or aerosolized delivery.
A variety of different antiviral strategies for MV inhibition have been considered, including antisense molecules, peptidic inhibitors, natural extracts, nucleoside analogs and small-molecule compounds. Vector-based antisense inhibitors [85,86] and peptide-conjugated morpholino oligomers [87], although effective in in vitro models of MV infection, have high production costs, limited storage stability, and delivery and bioavailability issues. Related concerns apply to peptidic inhibitors of MV entry, such as di- and tri-peptides (that are moderately effective in in vitro models of MV infection) [88,89] and highly potent peptides derived from the conserved heptad repeat B domain of the viral fusion protein [90]. The latter peptides act by a mechanism of membrane fusion inhibition considered analogous to that of the efficacious HIV-1 entry inhibitor enfuvirtide (Fuzeon) [91,92] and are potent, with active concentrations in the nanomolar range (EC50 = 0.1 μg/ml). However, because experience with the 36-residue peptide enfuvirtide has also highlighted several obstacles associated with heptad-repeat-derived peptidic antivirals (subcutaneous injection is required, injection-site reactions occur and the cost of therapy is approximately US $24,000 per year) [93], such inhibitors are unlikely to be a viable strategy for measles therapy and, therefore, have not been pursued for further development.
Multiple natural extracts or synthetic analogs derived from natural products have been reported to possess anti-MV activity [94-103]. However, many of these substances were only moderately active in cell culture, were cytotoxic, were inactive when added to cells post-exposure or the active ingredient remained elusive (reviewed in reference [104]).
Synthetic small-molecule compounds are by their nature likely to constitute the most suitable class of MV inhibitor because these compounds have a greater potential than other approaches to be mass produced cost effectively, to be stable at ambient temperature and to have desirable bioavailability. Table 1 provides an overview of the classes of MV inhibitor that were investigated previously or are currently under investigation. To minimize potential side effects, a pathogen-directed rather than host-directed antiviral strategy appears preferable for the treatment of measles. For instance, the entry and transcription/replication phase of the virus life cycle, driven by unique viral protein complexes that lack cellular homologs, constitute attractive targets. However, a pathogen-directed approach is at a greater risk for emerging viral resistance. While resistance to treatment is in general a particular challenge for the treatment of persistent viral infections, it must be investigated whether this applies equally to therapy for a pathogen such as MV that causes predominantly acute disease and induces strong immunity. Treatment-resistant variants may be of little clinical relevance if they coincide with reduced efficiency of viral transmission.
Table 1. Selected synthetic inhibitors with reported activity against MV.
| Compound or class | EC50 concentration | Specificity | Proposed target, if known | Minimal efficacious dose | Reference |
|---|---|---|---|---|---|
| Neplanocins | 0.1 to 1 μg/ml | Broad-range | S-adenosylhomocysteine hydrolase | ND | [108,109] |
| Noraristeromycins | 0.4 μg/ml | Broad-range | S-adenosylhomocysteine hydrolase | ND | [110,111] |
| 5′-Nor carbocyclic adenosine analogs | < 0.4 μg/ml | MV-specific | ND | ND | [112] |
| 5′-Fluoro-5′-deoxyaristeromycin | 13 μM | MV-specific | S-adenosylhomocysteine hydrolase | ND | [113] |
| 5-Ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide (EICAR) | 0.1 to 1 μg/ml | Broad-range | IMP dehydrogenase | 120 mg/kg bid | [109,114] |
| Ribavirin | 17.6 to 47 μg/ml | Broad-range | Lethal mutagenesis | 360 mg/kg bid | [114-116] |
| Ring-expanded nucleoside analogs | 2 to 10 μM | MV-specific | ND | ND | [117] |
| Mycophenolic acid | 0.2 μg/ml | Broad-range | IMP dehydrogenase | ND | [116] |
| Coumarin analogs | 0.2 to 50 μg/ml | MV specific | Possibly RdRp | ND | [118] |
| N-phosphonacetyl-l-aspartate (PALA) | 8 μg/ml | Broad-range | l-aspartic acid transcarbamoylase | ND | [119] |
| Pathogen-associated molecular patterns (PAMPs) | 0.5 μg/ml | Broad-range | Possibly virus attachment | No therapeutic effect | [114] |
| Isoquinolin analogs | 10 μM | MV-specific | ND | ND | [120] |
| AS-48 | 0.6 to 2 μM (wild-type isolates) | MV-specific | MV fusion protein | Inactive | [121-123] |
| Isothiazole analogs | 2.9 μg/ml | Broad-range | ND | ND | [124,125] |
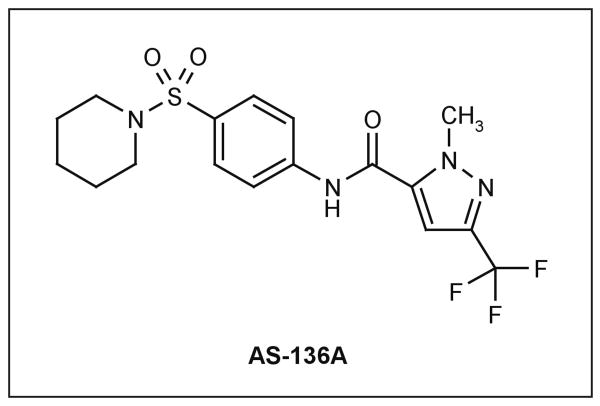
| AS-136A | 10 to 50 nM (wild-type isolates) | MV-specific | MV RdRp complex | ND | [105,106,126] |
When available, postulated targets of the different compounds, measles virus (MV)-specificity versus broad-range antiviral activity (viruses of the paramyxovirus and/or other virus families), and the minimal efficacious dose in the cotton rat model of MV infection are provided. IMP inosine monophosphate, ND not determined, RdRp RNA-dependent RNA polymerase
A newly developed class of non-nucleoside, target-specific inhibitors of MV polymerase complex activity demonstrated potent antiviral activity, in the nanomolar range, when tested against a panel of wild-type MV isolates representing currently endemic genotypes [105]. Combined with overall desirable pharmacological features of the scaffold (Figure 2), high chemical stability and low cytotoxicity (selectivity index CC50/EC50 ∼ 16,500 [106]), this class is an example of a novel developmental lead that is tailored to the specific requirements for a measles therapeutic.
Figure 2. The structure of AS-136A.
AS-136A is a specific inhibitor of measles virus RNA-dependent RNA polymerase activity with desirable pharmacological properties.
Conclusions
The Measles Initiative launched in 2001 has made impressive progress towards reducing the global morbidity and mortality caused by measles. This was achieved through a substantial increase in coverage with the live-attenuated measles vaccine, in particular in Africa, and the Eastern Mediterranean and Western Pacific regions [32]. Despite these achievements, the highest numbers of measles case in more than a decade were reported in 2008 for several industrialized countries, and the virus was again declared endemic in the UK. This resurgence, which results mostly from individual or parental reservations against vaccination based on philosophical or religious beliefs, highlights the challenges associated with a decline in public acceptance of vaccination [107]. Further complications arise from the limitations associated with the currently available vaccines as described in detail in the previous section.
In addition to improvements in the control of MV by new vaccines [51,53], the development of novel, safe and cost-effective small-molecule measles antivirals can contribute to overcoming these limitations. Rather than creating an alternative approach, MV inhibitors can be a useful addition to the prophylactic options available against MV, thereby providing a combined prophylactic and therapeutic anti-measles platform. Conceivable areas of immediate use of antivirals include acute and persistent disease (to improve case management), post-exposure prophylaxis, rapid control of local outbreaks before vaccinations become available or in cases of declined vaccination and protection of immunocompromised individuals and infants prior to vaccination. In the long term, antivirals could assist in a prolonged endgame of global eradication, as experienced with poliovirus.
Suitability for these applications sets clear parameters for ideal measles antivirals. The desired drug is safe and effective, mass producible at low cost, characterized by high shelf-stability at ambient temperature and is orally available. None of the experimental measles therapies tested clinically thus far matches this diverse array of requirements, necessitating de novo development. Small-molecule antiviral compounds are considered to be best suited to meeting these criteria. To date, promising chemical scaffolds have been identified experimentally that have demonstrated potent antiviral activity. Broadening the current drug discovery efforts and moving promising new hits and current leads through preclinical development is warranted to enable effective antivirals to become clinically available.
Acknowledgments
The research of the authors is supported in part by US Public Health Service Grant AI071002 (RKP) from the NIH/NIAID.
Contributor Information
Richard K Plemper, Email: rplempe@emory.edu.
James P Snyder, Email: jsnyder@emory.edu.
References
• of special interest
- 1.Lamb RA, Parks GD. Paramyxoviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology, 1. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2007. pp. 1449–1496. [Google Scholar]
- 2.Moss WJ, Griffin DE. Global measles elimination. Nat Rev Microbiol. 2006;4(12):900–908. doi: 10.1038/nrmicro1550. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An excellent overview of recent progress toward the control of MV.
- 3.Griffin DE. Measles virus. In: Knipe DM, Howley PM, editors. Fields Virology, 1. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2007. pp. 1551–1585. [Google Scholar]
- 4.Hethcote HW. The mathematics of infectious disease. SIAM Review. 2000;42(4):599–653. [Google Scholar]
- 5.Black FL. Measles endemicity in insular populations: Critical community size and its evolutionary implication. J Theor Biol. 1966;11(2):207–211. doi: 10.1016/0022-5193(66)90161-5. [DOI] [PubMed] [Google Scholar]
- 6.Keeling MJ. Modelling the persistence of measles. Trends Microbiol. 1997;5(12):513–518. doi: 10.1016/S0966-842X(97)01147-5. [DOI] [PubMed] [Google Scholar]
- 7.Compans RW, Mountcastle WE, Choppin PW. The sense of the helix of paramyxovirus nucleocapsids. J Mol Biol. 1972;65(1):167–169. doi: 10.1016/0022-2836(72)90499-8. [DOI] [PubMed] [Google Scholar]
- 8.Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406(6798):893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]; • The first identification of SLAM as a receptor for MV isolates.
- 9.Tatsuo H, Ono N, Yanagi Y. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J Virol. 2001;75(13):5842–5850. doi: 10.1128/JVI.75.13.5842-5850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naniche D, Varior-Krishnan G, Cervoni F, Wild TF, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67(10):6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manchester M, Eto DS, Valsamakis A, Liton PB, Fernandez-Muñoz R, Rota PA, Bellini WJ, Forthal DN, Oldstone MB. Clinical isolates of measles virus use CD46 as a cellular receptor. J Virol. 2000;74(9):3967–3974. doi: 10.1128/jvi.74.9.3967-3974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75(2):295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 13.Rima BK, Duprex WP. The measles virus replication cycle. Curr Top Microbiol Immunol. 2009;329:77–102. doi: 10.1007/978-3-540-70523-9_5. [DOI] [PubMed] [Google Scholar]
- 14.Sidhu MS, Chan J, Kaelin K, Spielhofer P, Radecke F, Schneider H, Masurekar M, Dowling PC, Billeter MA, Udem SA. Rescue of synthetic measles virus minireplicons: Measles genomic termini direct efficient expression and propagation of a reporter gene. Virology. 1995;208(2):800–807. doi: 10.1006/viro.1995.1215. [DOI] [PubMed] [Google Scholar]
- 15.de Swart RL. The pathogenesis of measles revisited. Pediatr Infect Dis J. 2008;27(10 Suppl):S84–S88. doi: 10.1097/INF.0b013e31816857fe. [DOI] [PubMed] [Google Scholar]
- 16.von Messling V, Milosevic D, Cattaneo R. Tropism illuminated: Lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc Natl Acad Sci USA. 2004;101(39):14216–14221. doi: 10.1073/pnas.0403597101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Swart RL, Ludlow M, de Witte L, Yanagi Y, van Amerongen G, McQuaid S, Yüksel S, Geijtenbeek TB, Duprex WP, Osterhaus AD. Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 2007;3(11):e178. doi: 10.1371/journal.ppat.0030178. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Identification of predominant infection of lymphoid cells in a nonhuman primate model.
- 18.Epidemiology and Prevention of Vaccine-Preventable Diseases. 11th. Public Health Foundation; Washington, DC, USA: 2008. [Google Scholar]
- 19.Hilleman MR. Current overview of the pathogenesis and prophylaxis of measles with focus on practical implications. Vaccine. 2001;20(56):651–665. doi: 10.1016/s0264-410x(01)00384-x. [DOI] [PubMed] [Google Scholar]
- 20.Nasr JT, Andriola MR, Coyle PK. ADEM: Literature review and case report of acute psychosis presentation. Pediatr Neurol. 2000;22(1):8–18. doi: 10.1016/s0887-8994(99)00116-2. [DOI] [PubMed] [Google Scholar]
- 21.Rima BK, Duprex WP. Morbilliviruses and human disease. J Pathol. 2006;208(2):199–214. doi: 10.1002/path.1873. [DOI] [PubMed] [Google Scholar]; • Comprehensive review of diseases that have been associated with MV.
- 22.Centers for Disease Control and Prevention (CDC) Progress in global measles control and mortality reduction, 2000-2007. MMWR Morb Mortal Wkly Rep. 2008;57(48):1303–1306. [PubMed] [Google Scholar]
- 23.Rosenau BJ, Schur PH. Association of measles virus with rheumatoid arthritis. J Rheumatol. 2009;36(5):893–897. doi: 10.3899/jrheum.080856. [DOI] [PubMed] [Google Scholar]
- 24.Tucker WG, Andrew Paskauskas R. The MSMV hypothesis: Measles virus and multiple sclerosis, etiology and treatment. Med Hypotheses. 2008;71(5):682–689. doi: 10.1016/j.mehy.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 25.Ohara Y. Multiple sclerosis and measles virus. Jpn J Infect Dis. 1999;52(5):198–200. [PubMed] [Google Scholar]
- 26.Cosby SL, McQuaid S, Taylor MJ, Bailey M, Rima BK, Martin SJ, Allen IV. Examination of eight cases of multiple sclerosis and 56 neurological and non-neurological controls for genomic sequences of measles virus, canine distemper virus, simian virus 5 and rubella virus. J Gen Virol. 1989;70(Pt 8):2027–2036. doi: 10.1099/0022-1317-70-8-2027. [DOI] [PubMed] [Google Scholar]
- 27.Ledford H. Viruses found in lung tumours. Nature Publishing Group; London, UK: 2008. www.nature.com/news/2008/080425/full/news.2008.779.html. [Google Scholar]
- 28.Benharroch D, Shemer-Avni Y, Myint YY, Levy A, Mejirovsky E, Suprun I, Shendler Y, Prinsloo I, Ariad S, Rager-Zisman B, Sacks M, et al. Measles virus: Evidence of an association with Hodgkin's disease. Br J Cancer. 2004;91(3):572–579. doi: 10.1038/sj.bjc.6601900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benharroch D, Shemer-Avni Y, Levy A, Myint YY, Ariad S, Rager B, Sacks M, Gopas J. New candidate virus in association with Hodgkin's disease. Leuk Lymphoma. 2003;44(4):605–610. doi: 10.1080/1042819021000037994. [DOI] [PubMed] [Google Scholar]
- 30.Orenstein WA, Strebel PM, Papania M, Sutter RW, Bellini WJ, Cochi SL. Measles eradication: Is it in our future. Am J Public Health. 2000;90(10):1521–1525. doi: 10.2105/ajph.90.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Excellent early discussion of the feasibliy of the global eradication of MV.
- 31.Centers for Disease Control and Prevention (CDC) Update: Global measles control and mortality reduction – Worldwide, 1991-2001. MMWR Morb Mortal Wkly Rep. 2003;52(20):471–475. [PubMed] [Google Scholar]
- 32.From the Centers for Disease Control and Prevention: Progress in global measles control and mortality reduction, 2000-2006. J Am Med Assoc. 2008;299(4):400–402. [Google Scholar]
- 33.de Quadros CA, Andrus JK, Danovaro-Holliday MC, Castillo-Solorzano C. Feasibility of global measles eradication after interruption of transmission in the Americas. Expert Rev Vaccines. 2008;7(3):355–362. doi: 10.1586/14760584.7.3.355. [DOI] [PubMed] [Google Scholar]
- 34.Wolfson LJ, Strebel PM, Gacic-Dobo M, Hoekstra EJ, McFarland JW, Hersh BS. Has the 2005 measles mortality reduction goal been achieved? A natural history modelling study. Lancet. 2007;369(9557):191–200. doi: 10.1016/S0140-6736(07)60107-X. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC) Update: Measles - United States, January-July 2008. MMWR Morb Mortal Wkly Rep. 2008;57(33):893–896. [PubMed] [Google Scholar]
- 36.Kremer JR, Muller CP. Measles in Europe - there is room for improvement. Lancet. 2009;373(9661):356–358. doi: 10.1016/S0140-6736(08)61850-4. [DOI] [PubMed] [Google Scholar]
- 37.EUVAC.NET; Copenhagen, Denmark: 2008. Measles surveillance first quarterly report 2008. www.euvac.net/graphics/euvac/pdf/2008_first.pdf. [Google Scholar]
- 38.EUVAC.NET; Copenhagen, Denmark: 2008. Measles surveillance second quarterly report 2008. www.euvac.net/graphics/euvac/pdf/2008_second.pdf. [Google Scholar]
- 39.Health Protection Agency; London, UK: 2008. Confirmed measles cases in England and Wales – an update to the end of October 2008. Health Protection Report. www.hpa.org.uk/hpr/archives/2008/news4808.htm#measles. [Google Scholar]
- 40.Muscat M, Bang H, Wohlfahrt J, Glismann S, Mølbak K. Measles in Europe: An epidemiological assessment. Lancet. 2009;373(9661):383–389. doi: 10.1016/S0140-6736(08)61849-8. [DOI] [PubMed] [Google Scholar]
- 41.Measles once again endemic in the United Kingdom. Eurosurveillance. 2008;13(27):3. [PubMed] [Google Scholar]
- 42.Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM, Malik M, Berelowitz M, Dhillon AP, Thomson MA, Harvey P, Valentine A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351(9103):637–641. doi: 10.1016/s0140-6736(97)11096-0. [DOI] [PubMed] [Google Scholar]
- 43.Madsen KM, Hviid A, Vestergaard M, Schendel D, Wohlfahrt J, Thorsen P, Olsen J, Melbye M. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med. 2002;347(19):1477–1482. doi: 10.1056/NEJMoa021134. [DOI] [PubMed] [Google Scholar]
- 44.DeStefano F, Thompson WW. MMR vaccine and autism: An update of the scientific evidence. Expert Rev Vaccines. 2004;3(1):19–22. doi: 10.1586/14760584.3.1.19. [DOI] [PubMed] [Google Scholar]
- 45.Murch SH, Anthony A, Casson DH, Malik M, Berelowitz M, Dhillon AP, Thomson MA, Valentine A, Davies SE, Walker-Smith JA. Retraction of an interpretation. Lancet. 2004;363(9411):750. doi: 10.1016/S0140-6736(04)15715-2. [DOI] [PubMed] [Google Scholar]
- 46.Meissner HC, Strebel PM, Orenstein WA. Measles vaccines and the potential for worldwide eradication of measles. Pediatrics. 2004;114(4):1065–1069. doi: 10.1542/peds.2004-0440. [DOI] [PubMed] [Google Scholar]
- 47.Gay NJ. The theory of measles elimination: Implications for the design of elimination strategies. J Infect Dis. 2004;189(Suppl 1):S27–S35. doi: 10.1086/381592. [DOI] [PubMed] [Google Scholar]
- 48.Watson JC, Hadler SC, Dykewicz CA, Reef S, Phillips L. Measles, mumps, and rubella – Vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1998;47(RR8):1–57. [PubMed] [Google Scholar]
- 49.de Quadros CA, Olivé JM, Hersh BS, Strassburg MA, Henderson DA, Brandling-Bennett D, Alleyne GA. Measles elimination in the Americas. Evolving strategies. J Am Med Assoc. 1996;275(3):224–229. doi: 10.1001/jama.275.3.224. [DOI] [PubMed] [Google Scholar]
- 50.Henao-Restrepo AM, Strebel P, John Hoekstra E, Birmingham M, Bilous J. Experience in global measles control, 1990-2001. J Infect Dis. 2003;187(Suppl 1):S15–S21. doi: 10.1086/368273. [DOI] [PubMed] [Google Scholar]
- 51.de Vries RD, Stittelaar KJ, Osterhaus AD, de Swart RL. Measles vaccination: New strategies and formulations. Expert Rev Vaccines. 2008;7(8):1215–1223. doi: 10.1586/14760584.7.8.1215. [DOI] [PubMed] [Google Scholar]; • Overview of goals and strategies for improved measles vaccines.
- 52.Gans HA, Arvin AM, Galinus J, Logan L, DeHovitz R, Maldonado Y. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. J Am Med Assoc. 1998;280(6):527–532. doi: 10.1001/jama.280.6.527. [DOI] [PubMed] [Google Scholar]
- 53.Griffin DE, Pan CH. Measles: Old vaccines, new vaccines. Curr Top Microbiol Immunol. 2009;330:191–212. doi: 10.1007/978-3-540-70617-5_10. [DOI] [PubMed] [Google Scholar]; • Overview of goals and strategies for improved vaccines for MV.
- 54.Pütz MM, Bouche FB, de Swart RL, Muller CP. Experimental vaccines against measles in a world of changing epidemiology. Int J Parasitol. 2003;33(56):525–545. doi: 10.1016/s0020-7519(03)00062-6. [DOI] [PubMed] [Google Scholar]
- 55.Mossong J, Nokes DJ, Edmunds WJ, Cox MJ, Ratnam S, Muller CP. Modeling the impact of subclinical measles transmission in vaccinated populations with waning immunity. Am J Epidemiol. 1999;150(11):1238–1249. doi: 10.1093/oxfordjournals.aje.a009951. [DOI] [PubMed] [Google Scholar]
- 56.Mossong J, O'Callaghan CJ, Ratnam S. Modelling antibody response to measles vaccine and subsequent waning of immunity in a low exposure population. Vaccine. 2000;19(45):523–529. doi: 10.1016/s0264-410x(00)00175-4. [DOI] [PubMed] [Google Scholar]
- 57.Guris D, McCready J, Watson JC, Atkinson WL, Heath JL, Bellini WJ, Polloi A. Measles vaccine effectiveness and duration of vaccine-induced immunity in the absence of boosting from exposure to measles virus. Pediatr Infect Dis J. 1996;15(12):1082–1086. doi: 10.1097/00006454-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J. 1990;9(2):101–110. doi: 10.1097/00006454-199002000-00008. [DOI] [PubMed] [Google Scholar]
- 59.Fernandez H, Banks G, Smith R. Ribavirin: A clinical overview. Eur J Epidemiol. 1986;2(1):1–14. doi: 10.1007/BF00152711. [DOI] [PubMed] [Google Scholar]
- 60.Stogner SW, King JW, Black-Payne C, Bocchini J. Ribavirin and intravenous immune globulin therapy for measles pneumonia in HIV infection. South Med J. 1993;86(12):1415–1418. doi: 10.1097/00007611-199312000-00023. [DOI] [PubMed] [Google Scholar]
- 61.Gururangan S, Stevens RF, Morris DJ. Ribavirin response in measles pneumonia. J Infect. 1990;20(3):219–221. doi: 10.1016/0163-4453(90)91094-t. [DOI] [PubMed] [Google Scholar]
- 62.Pan American Health Organization. Measles Eradication: Field Guide. Pan American Health Organization; Washington, DC, USA: 1999. [Google Scholar]
- 63.Moss WJ, Cutts F, Griffin DE. Implications of the human immunodeficiency virus epidemic for control and eradication of measles. Clin Infect Dis. 1999;29(1):106–112. doi: 10.1086/520136. [DOI] [PubMed] [Google Scholar]
- 64.Permar SR, Moss WJ, Ryon JJ, Monze M, Cutts F, Quinn TC, Griffin DE. Prolonged measles virus shedding in human immunodeficiency virus-infected children, detected by reverse transcriptase-polymerase chain reaction. J Infect Dis. 2001;183(4):532–538. doi: 10.1086/318533. [DOI] [PubMed] [Google Scholar]
- 65.Roberts L. Polio eradication. Looking for a little luck. Science. 2009;323(5915):702–705. doi: 10.1126/science.323.5915.702. [DOI] [PubMed] [Google Scholar]
- 66.Katz SL. Polio - new challenges in 2006. J Clin Virol. 2006;36(3):163–165. doi: 10.1016/j.jcv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 67.Centers for Disease Control and Prevention; Atlanta, GA, USA: 2009. History and epidemiology of global smallpox eradication. www.bt.cdc.gov/agent/smallpox/training/overview/pdf/eradicationhistory.pdf. [Google Scholar]
- 68.Lecciones JA, Abejar NH, Dimaano EE, Bartolome R, Cinco S, Mariano N, Yerro ME, Cobar S, Fuggan B. A pilot double-blind, randomized, and placebo-controlled study of orally administered IFN-α-n1 (Ins) in pediatric patients with measles. J Interferon Cytokine Res. 1998;18(9):647–652. doi: 10.1089/jir.1998.18.647. [DOI] [PubMed] [Google Scholar]
- 69.Gascon GG. Randomized treatment study of inosiplex versus combined inosiplex and intraventricular interferon-α in subacute sclerosing panencephalitis (SSPE): International multicenter study. J Child Neurol. 2003;18(12):819–827. doi: 10.1177/088307380301801201. [DOI] [PubMed] [Google Scholar]
- 70.Hosoya M. Therapy and prognosis in subacute sclerosing panencephalitis. Nippon Rinsho. 2007;65(8):1483–1486. [PubMed] [Google Scholar]
- 71.Miyazaki M, Nishimura M, Toda Y, Saijo T, Mori K, Kuroda Y. Long-term follow-up of a patient with subacute sclerosing panencephalitis successfully treated with intrathecal interferon α. Brain Dev. 2005;27(4):301–303. doi: 10.1016/j.braindev.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Hara S, Kimura H, Hoshino Y, Hayashi N, Negoro T, Okumura A, Kajita Y, Sakuma T, Nakayama T, Hosoya M, Tomoda A, et al. Combination therapy with intraventricular interferon-α and ribavirin for subacute sclerosing panencephalitis and monitoring measles virus RNA by quantitative PCR assay. Brain Dev. 2003;25(5):367–369. doi: 10.1016/s0387-7604(03)00017-2. [DOI] [PubMed] [Google Scholar]
- 73.del Toro-Riera M, Macaya-Ruiz A, Raspall-Chaure M, Tallada-Serra M, Pasqual-López I, Roig-Quilis M. Subacute sclerosing panencephalitis: Combined treatment with interferon α and intraventricular ribavirin. Rev Neurol. 2006;42(5):277–281. [PubMed] [Google Scholar]
- 74.Campbell C, Levin S, Humphreys P, Walop W, Brannan R. Subacute sclerosing panencephalitis: Results of the Canadian Paediatric Surveillance Program and review of the literature. BMC Pediatr. 2005;5:47. doi: 10.1186/1471-2431-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oshiro S, Minema H, Shiroma N, Hirayasu K, Nakada Y. Five patients with subacute sclerosing panencephalitis treated with intraventricular α-interferon and inosinpranobex. No To Hattatsu. 2004;36(1):70–74. [PubMed] [Google Scholar]
- 76.Gagnon A, Bouchard RW. Fulminating adult-onset subacute sclerosing panencephalitis in a 49-year-old man. Arch Neurol. 2003;60(8):1160–1161. doi: 10.1001/archneur.60.8.1160. [DOI] [PubMed] [Google Scholar]
- 77.Tomoda A, Nomura K, Shiraishi S, Miike T, Hamada A, Hosoya M. Trial of intraventricular ribavirin and interferon-α combination therapy for subacute sclerosing panencephalitis (SSPE) in Japan. No To Hattatsu. 2003;35(4):321–326. [PubMed] [Google Scholar]
- 78.Forni AL, Schluger NW, Roberts RB. Severe measles pneumonitis in adults: Evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin Infect Dis. 1994;19(3):454–462. doi: 10.1093/clinids/19.3.454. [DOI] [PubMed] [Google Scholar]
- 79.Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: Efficacy, side effects, and complications. Gut. 2006;55(9):1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. PEGinterferon α-2b plus ribavirin compared with interferon α-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358(9286):958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 81.Barclay AJ, Foster A, Sommer A. Vitamin A supplements and mortality related to measles: A randomised clinical trial. Br Med J (Clin Res Ed) 1987;294(6567):294–296. doi: 10.1136/bmj.294.6567.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.D'Souza RM, D'Souza R. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2002;(1) doi: 10.1002/14651858.CD001479. CD001479. [DOI] [PubMed] [Google Scholar]
- 83.Villamor E, Fawzi WW. Vitamin A supplementation: Implications for morbidity and mortality in children. J Infect Dis. 2000;182(Suppl 1):S122–S133. doi: 10.1086/315921. [DOI] [PubMed] [Google Scholar]
- 84.Wyde PR. Chemotherapy of respiratory viruses: Prospects and challenges. Drug Resist Updat. 1999;2(4):244–258. doi: 10.1054/drup.1999.0091. [DOI] [PubMed] [Google Scholar]
- 85.Koschel K, Brinckmann U, Hoyningen-Huene VV. Measles virus antisense sequences specifically cure cells persistently infected with measles virus. Virology. 1995;207(1):168–178. doi: 10.1006/viro.1995.1063. [DOI] [PubMed] [Google Scholar]
- 86.Bell AF, Whitton JL, Fujinami RS. Antisense-mediated resistance to measles virus infection in HeLa cells. J Infect Dis. 1997;176(1):258–261. doi: 10.1086/517261. [DOI] [PubMed] [Google Scholar]
- 87.Sleeman K, Stein DA, Tamin A, Reddish M, Iversen PL, Rota PA. Inhibition of measles virus infections in cell cultures by peptide-conjugated morpholino oligomers. Virus Res. 2009;140(12):49–56. doi: 10.1016/j.virusres.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 88.Miller FA, Dixon GJ, Arnett G, Dice JR, Rightsel WA, Schabel FM, Jr, McLean IW., Jr Antiviral activity of carbobenzosy di- and tripeptides on measles virus. Appl Microbiol. 1968;16(10):1489–1496. doi: 10.1128/am.16.10.1489-1496.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nicolaides E, DeWald H, Lipnik M, Westland R, Posler J. Potential antiviral agents. Carbobenzoxy di- and tripeptides active against measles and herpes viruses. J Med Chem. 1968;11(1):74–79. doi: 10.1021/jm00307a016. [DOI] [PubMed] [Google Scholar]
- 90.Lambert DM, Barney S, Lambert AL, Guthrie K, Medinas R, Davis DE, Bucy T, Erickson J, Merutka G, Petteway SR., Jr Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc Natl Acad Sci USA. 1996;93(5):2186–2191. doi: 10.1073/pnas.93.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Identification of peptidic inhibitors against different paramyxoviruses.
- 91.Kilby JM, Hopkins S, Venetta TM, DiMassimo B, Cloud GA, Lee JY, Alldredge L, Hunter E, Lambert D, Bolognesi D, Matthews T, et al. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med. 1998;4(11):1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]; • Demonstration of efficacy of peptidic (heptad repeat-derived) inhibitors of HIV.
- 92.Kilby JM, Lalezari JP, Eron JJ, Carlson M, Cohen C, Arduino RC, Goodgame JC, Gallant JE, Volberding P, Murphy RL, Valentine F, et al. The safety, plasma pharmacokinetics, and antiviral activity of subcutaneous enfuvirtide (T-20), a peptide inhibitor of gp41-mediated virus fusion, in HIV-infected adults. AIDS Res Hum Retroviruses. 2002;18(10):685–693. doi: 10.1089/088922202760072294. [DOI] [PubMed] [Google Scholar]
- 93.Jamjian MC, McNicholl IR. Enfuvirtide: First fusion inhibitor for treatment of HIV infection. Am J Health Syst Pharm. 2004;61(12):1242–1247. doi: 10.1093/ajhp/61.12.1242. [DOI] [PubMed] [Google Scholar]
- 94.Parker ME, Chabot S, Ward BJ, Johns T. Traditional dietary additives of the Maasai are antiviral against the measles virus. J Ethnopharmacol. 2007;114(2):146–152. doi: 10.1016/j.jep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 95.Petricevich VL, Mendonça RZ. Inhibitory potential of Crotalus durissus terrificus venom on measles virus growth. Toxicon. 2003;42(2):143–153. doi: 10.1016/s0041-0101(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 96.Wachsman MB, Ramirez JA, Galagovsky LR, Coto CE. Antiviral activity of brassinosteroids derivatives against measles virus in cell cultures. Antivir Chem Chemother. 2002;13(1):61–66. doi: 10.1177/095632020201300105. [DOI] [PubMed] [Google Scholar]
- 97.Olila D, Olwa O, Opuda-Asibo J. Screening extracts of Zanthoxylum chalybeum and Warburgia ugandensis for activity against measles virus (Swartz and Edmonston strains) in vitro. Afr Health Sci. 2002;2(1):2–10. [PMC free article] [PubMed] [Google Scholar]
- 98.Cos P, Hermans N, De Bruyne T, Apers S, Sindambiwe JB, Vanden Berghe D, Pieters L, Vlietinck AJ. Further evaluation of Rwandan medicinal plant extracts for their antimicrobial and antiviral activities. J Ethnopharmacol. 2002;79(2):155–163. doi: 10.1016/s0378-8741(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 99.Huang SP, Shieh GJ, Lee L, Teng HJ, Kao ST, Lin JG. Inhibition effect of shengma-gegen-tang on measles virus in Vero cells and human peripheral blood mononuclear cells. Am J Chin Med. 1997;25(1):89–96. doi: 10.1142/S0192415X97000123. [DOI] [PubMed] [Google Scholar]
- 100.McWhorter JH. Spicebush. A Cherokee remedy for the measles. N C Med J. 1996;57(5):306. [PubMed] [Google Scholar]
- 101.Lin YM, Flavin MT, Schure R, Chen FC, Sidwell R, Barnard DL, Huffman JH, Kern ER. Antiviral activities of biflavonoids. Planta Med. 1999;65(2):120–125. doi: 10.1055/s-1999-13971. [DOI] [PubMed] [Google Scholar]
- 102.Kurokawa M, Ochiai H, Nagasaka K, Neki M, Xu H, Kadota S, Sutardjo S, Matsumoto T, Namba T, Shiraki K. Antiviral traditional medicines against herpes simplex virus (HSV-1), poliovirus, and measles virus in vitro and their therapeutic efficacies for HSV-1 infection in mice. Antiviral Res. 1993;22(23):175–188. doi: 10.1016/0166-3542(93)90094-y. [DOI] [PubMed] [Google Scholar]
- 103.Hayashi T, Hayashi K, Maeda M, Kojima I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J Nat Prod. 1996;59(1):83–87. doi: 10.1021/np960017o. [DOI] [PubMed] [Google Scholar]
- 104.Barnard DL. Inhibitors of measles virus. Antivir Chem Chemother. 2004;15(3):111–119. doi: 10.1177/095632020401500301. [DOI] [PubMed] [Google Scholar]; • Comprehensive review of experimental MV inhibitors.
- 105.White LK, Yoon JJ, Lee JK, Sun A, Du Y, Fu H, Snyder JP, Plemper RK. Nonnucleoside inhibitor of measles virus RNA-dependent RNA polymerase complex activity. Antimicrob Agents Chemother. 2007;51(7):2293–2303. doi: 10.1128/AAC.00289-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First report of a highly potent small-molecule MV RdRp inhibitor class.
- 106.Sun A, Chandrakumar N, Yoon JJ, Plemper RK, Snyder JP. Non-nucleoside inhibitors of the measles virus RNA-dependent RNA polymerase complex activity: Synthesis and in vitro evaluation. Bioorg Med Chem Lett. 2007;17(18):5199–5203. doi: 10.1016/j.bmcl.2007.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Offit PA, editor. Autism's False Prophets Bad Science, Risky Medicine and the Search for a Cure. Columbia University Press; New York, NY, USA: 2008. [Google Scholar]
- 108.Shuto S, Obara T, Toriya M, Hosoya M, Snoeck R, Andrei G, Balzarini J, De Clercq E. New neplanocin analogues. 1. Synthesis of 6′-modified neplanocin A derivatives as broad-spectrum antiviral agents. J Med Chem. 1992;35(2):324–331. doi: 10.1021/jm00080a018. [DOI] [PubMed] [Google Scholar]
- 109.Shigeta S, Mori S, Baba M, Ito M, Honzumi K, Nakamura K, Oshitani H, Numazaki Y, Matsuda A, Obara T, et al. Antiviral activities of ribavirin, 5-ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide, and 6′-(R)-6′-C-methylneplanocin A against several ortho- and paramyxoviruses. Antimicrob Agents Chemother. 1992;36(2):435–439. doi: 10.1128/aac.36.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patil SD, Schneller SW, Hosoya M, Snoeck R, Andrei G, Balzarini J, De Clercq E. Synthesis and antiviral properties of (±)-5′-noraristeromycin and related purine carbocyclic nucleosides. A new lead for anti-human cytomegalovirus agent design. J Med Chem. 1992;35(18):3372–3377. doi: 10.1021/jm00096a012. [DOI] [PubMed] [Google Scholar]
- 111.Siddiqi SM, Chen X, Rao J, Schneller SW, Ikeda S, Snoeck R, Andrei G, Balzarini J, De Clercq E. 3-deaza- and 7-deaza-5′-noraristeromycin and their antiviral properties. J Med Chem. 1995;38(6):1035–1038. doi: 10.1021/jm00006a023. [DOI] [PubMed] [Google Scholar]
- 112.Barnard DL, Stowell VD, Seley KL, Hegde VR, Das SR, Rajappan VP, Schneller SW, Smee DF, Sidwell RW. Inhibition of measles virus replication by 5′-nor carbocyclic adenosine analogues. Antivir Chem Chemother. 2001;12(4):241–250. doi: 10.1177/095632020101200405. [DOI] [PubMed] [Google Scholar]
- 113.Li W, Yin X, Schneller SW. 5′-Fluoro-5′-deoxyaristeromycin. Bioorg Med Chem Lett. 2008;18(1):220–222. doi: 10.1016/j.bmcl.2007.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wyde PR, Moore-Poveda DK, De Clercq E, Neyts J, Matsuda A, Minakawa N, Guzman E, Gilbert BE. Use of cotton rats to evaluate the efficacy of antivirals in treatment of measles virus infections. Antimicrob Agents Chemother. 2000;44(5):1146–1152. doi: 10.1128/aac.44.5.1146-1152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Crotty S, Maag D, Arnold JJ, Zhong W, Lau JY, Hong Z, Andino R, Cameron CE. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat Med. 2000;6(12):1375–1379. doi: 10.1038/82191. [DOI] [PubMed] [Google Scholar]
- 116.Kosugi Y, Saito Y, Mori S, Watanabe J, Baba M, Shigeta S. Antiviral activies of mizoribine and other inosine monophosphate dehydrogenase inhibitors against several ortho-and paramyxoviruses. Antivir Chem Chemother. 1994;5(6):366–371. [Google Scholar]
- 117.Zhang N, Chen HM, Sood R, Kalicharran K, Fattom AI, Naso RB, Barnard DL, Sidwell RW, Hosmane RS. In vitro inhibition of the measles virus by novel ring-expanded (‘fat’) nucleoside analogues containing the imidazo[4,5-e]diazepine ring system. Bioorg Med Chem Lett. 2002;12(23):3391–3394. doi: 10.1016/s0960-894x(02)00762-x. [DOI] [PubMed] [Google Scholar]
- 118.Barnard DL, Xu ZQ, Stowell VD, Yuan H, Smee DF, Samy R, Sidwell RW, Nielsen MK, Sun L, Cao H, Li A, et al. Coumarins and pyranocoumarins, potential novel pharmacophores for inhibition of measles virus replication. Antivir Chem Chemother. 2002;13(1):39–59. doi: 10.1177/095632020201300104. [DOI] [PubMed] [Google Scholar]
- 119.Wyde PR, Moore DK, Pimentel DM, Blough HA. Evaluation of the antiviral activity of N-(phosphonoacetyl)-l-aspartate against paramyxoviruses in tissue culture and against respiratory syncytial virus in cotton rats. Antiviral Res. 1995;27(12):59–69. doi: 10.1016/0166-3542(94)00080-r. [DOI] [PubMed] [Google Scholar]
- 120.Santagati NA, Bousquet E, Garozzo A, Prezzavento O, Spadaro A, Ronsisvalle G. Synthesis and anti-measles virus activity of new isoquinolin-4-one derivatives. Farmaco. 2003;58(12):1217–1225. doi: 10.1016/j.farmac.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 121.Plemper RK, Doyle J, Sun A, Prussia A, Cheng LT, Rota PA, Liotta DC, Snyder JP, Compans RW. Design of a small-molecule entry inhibitor with activity against primary measles virus strains. Antimicrob Agents Chemother. 2005;49(9):3755–3761. doi: 10.1128/AAC.49.9.3755-3761.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Plemper RK, Erlandson KJ, Lakdawala AS, Sun A, Prussia A, Boonsombat J, Aki-Sener E, Yalcin I, Yildiz I, Temiz-Arpaci O, Tekiner B, et al. A target site for template-based design of measles virus entry inhibitors. Proc Natl Acad Sci USA. 2004;101(15):5628–5633. doi: 10.1073/pnas.0308520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun A, Prussia A, Zhan W, Murray EE, Doyle J, Cheng LT, Yoon JJ, Radchenko EV, Palyulin VA, Compans RW, Liotta DC, et al. Nonpeptide inhibitors of measles virus entry. J Med Chem. 2006;49(17):5080–5092. doi: 10.1021/jm0602559. [DOI] [PubMed] [Google Scholar]
- 124.Garozzo A, Cutri CC, Pannecouque C, Castro A, Guerrera F, De Clercq E. Isothiazole derivatives as antiviral agents. Antivir Chem Chemother. 2007;18(5):277–283. doi: 10.1177/095632020701800503. [DOI] [PubMed] [Google Scholar]
- 125.Cutrì CC, Garozzo A, Siracusa MA, Castro A, Tempera G, Sarvà MC, Guerrera F. Synthesis of new 3-methylthio-5-aryl-4-isothiazolecarbonitriles with broad antiviral spectrum. Antiviral Res. 2002;55(2):357–368. doi: 10.1016/s0166-3542(02)00072-4. [DOI] [PubMed] [Google Scholar]
- 126.Sun A, Yoon JJ, Yin Y, Prussia A, Yang Y, Min J, Plemper RK, Snyder JP. Potent non-nucleoside inhibitors of the measles virus RNA-dependent RNA polymerase complex. J Med Chem. 2008;51(13):3731–3741. doi: 10.1021/jm701239a. [DOI] [PMC free article] [PubMed] [Google Scholar]