Table 1.
Synthesis and in vitro reactivation potency of monoquaternary pyridinium oximes 5–35 for paraoxon-inhibited EeAChE and rHuAChE
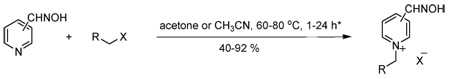 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | R | Oxime position |
X | Reaction time (h) |
% Yield |
% Reactivation = [(Ar-Ai)/(Ao-Ai)] × 100 | |||||
| EeAChE (% R ± SEM) | rHuAChE (% R ± SEM) | ||||||||||
| 10−3 Ma | 10−4 Ma | 10−5 Ma | 10−3 Ma | 10−4 Ma | 10−5 Ma | ||||||
| 1 | H | 2 | I | - | - | 67 ± 1 | 67 ± 3 | 63 ± 3 | 42 ± 2 | 22 ± 2 | 7 ± 1 |
| 5 | CONH2 | 2 | I | 24 | 62 | 41 ± 3 | 9 ± 0 | 1 ± 0 | 2 ± 1 | 2 ± 0 | 1 ± 0 |
| 6 | CONH2 | 3 | I | 2 | 76 | 1 ± 1 | 0 | 0 | 0 | 1 ± 0 | 2 ± 0 |
| 7 | CONH2 | 4 | I | 2 | 84 | 44 ± 2 | 6 ± 1 | 0 | 0 | 1 ± 0 | 1 ± 0 |
| 8 | COOH | 2 | I | 24 | 61 | 45 ± 3 | 36 ± 3 | 34 ± 2 | 15 ± 1 | 15 ± 2 | 5 ± 1 |
| 9 | COOH | 3 | I | 2 | 71 | 1 ± 0 | 0 | 0 | 0 | 1 ± 0 | 1 ± 0 |
| 10 | COOH | 4 | I | 2 | 78 | 4 ± 0 | 0 | 0 | 0 | 1 ± 0 | 2 ± 0 |
| 11 | CH2OH | 2 | Br | 24 | 58 | 50 ± 4 | 37 ± 3 | 13 ± 1 | 2 ± 2 | 5 ± 0 | 2 ± 0 |
| 12 | CH2OH | 3 | Br | 2 | 60 | 8 ± 1 | 0 | 0 ± 0 | 0 | 1 ± 0 | 1 ± 0 |
| 13 | CH2OH | 4 | Br | 2 | 68 | 70 ± 2 | 2 ± 2 | 3 ± 1 | 6 ± 1 | 3 ± 0 | 2 ± 0 |
| 14 | CH2COOH | 2 | Br | 24 | 48 | 10 ±1 | 0 | 0 | 0 | 1 ± 0 | 1 ± 0 |
| 15 | CH2COOH | 3 | Br | 2 | 56 | 5 ± 0 | 0 | 0 | 0 | 1 ± 0 | 1 ± 0 |
| 16 | CH2COOH | 4 | Br | 2 | 68 | 15 ±1 | 0 | 0 | 0 | 2 ± 0 | 1 ± 0 |
| 17 | 4-methoxybenzen-1-yl | 2 | Cl | 12 | 70 | 8 ± 1 | 19 ± 2 | 18 ± 2 | 0 | 7 ± 1 | 3 ± 0 |
| 18 | 4-methoxybenzen-1-yl | 3 | Cl | 1 | 84 | 1 ± 1 | 0 | 0 | 0 | 3 ± 0 | 2 ± 0 |
| 19 | 4-methoxybenzen-1-yl | 4 | Cl | 1 | 92 | 19 ± 1 | 11 ± 1 | 3 ± 1 | 0 | 3 ± 1 | 2 ± 0 |
| 20 | thiophen-2-yl | 2 | Cl | 24 | 58 | 1 ± 0 | 24 ± 2 | 34 ± 2 | 0 | 9 ± 2 | 9 ± 1 |
| 21 | thiophen-2-yl | 3 | Cl | 2 | 65 | 0 | 3 ± 1 | 1 ± 0 | 0 | 4 ± 0 | 5 ± 0 |
| 22 | thiophen-2-yl | 4 | Cl | 2 | 78 | 3 ± 0 | 12 ± 2 | 12 ± 0 | 0 | 4 ± 1 | 5 ± 0 |
| 23 | 5-bromothiophen-2-yl | 2 | Cl | 12 | 74 | 0 | 29 ± 2 | 37 ± 2 | 0 | 7 ± 0 | 8 ± 1 |
| 24 | 5-bromothiophen-2-yl | 3 | Cl | 1 | 84 | 4 ± 1 | 7 ± 0 | 8 ± 1 | 0 | 5 ± 1 | 6 ± 0 |
| 25 | 5-bromothiophen-2-yl | 4 | Cl | 1 | 90 | 3 ± 1 | 14 ± 1 | 10 ± 1 | 0 | 5 ± 1 | 4 ± 0 |
| 26 | benzthiophen-3-yl | 2 | Cl | 24 | 67 | 0 | 28 ± 2 | 24 ± 3 | 0 | 7 ± 1 | 8 ± 1 |
| 27 | benzthiophen-3-yl | 3 | Cl | 2 | 79 | 0 | 5 ± 1 | 8 ± 1 | 0 | 2 ± 0 | 5 ± 1 |
| 28 | benzthiophen-3-yl | 4 | Cl | 2 | 85 | 0 | 0 | 1 ± 1 | 0 | 2 ± 0 | 2 ± 0 |
| 29 | thiophen-3-yl | 2 | Cl | 24 | 79 | 0 | 8 ± 1 | 28 ± 2 | 0 | 2 ± 0 | 5 ± 1 |
| 30 | furan-2-yl | 3 | Cl | 1 | 62 | 0 | 0 | 0 | 0 | 2 ± 0 | 2 ± 0 |
| 31 | furan-2-yl | 4 | Cl | 1 | 60 | 8 ± 1 | 14 ± 2 | 1 ± 1 | 0 | 3 ± 0 | 2 ± 0 |
| 32 | furan-3-yl | 3 | Cl | 1 | 46 | 0 | 0 | 0 | 0 | 3 ± 0 | 2 ± 0 |
| 33 | 3-methyl-isoxazol-5-yl | 2 | Br | 24 | 40 | 7 ± 1 | 25 ± 3 | 8 ± 1 | 0 | 7 ± 1 | 3 ± 0 |
| 34 | 3-methyl-isoxazol-5-yl | 3 | Br | 2 | 64 | 0 | 0 | 0 | 0 | 3 ± 1 | 2 ± 0 |
| 35 | 3-methyl-isoxazol-5-yl | 4 | Br | 2 | 67 | 20 ± 2 | 24 ± 2 | 6 ± 0 | 0 | 2 ± 1 | 2 ± 0 |
compounds 5–16 acetone, 60–70 °C, 2–24 h; 17–35 MeCN, 70–80 °C, 2–24 h;
reactivator concentration
