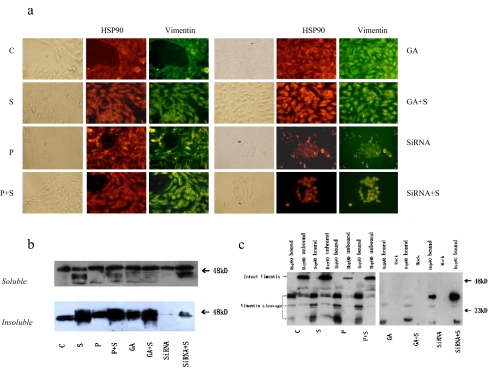
Fig. 3.
Identification of vimentin cleavage fragments as HSP90 binding protein. a Subcellular colocalization of vimentin and Hsp90. Cells were doubly stained for vimentin and Hsp90. b Adherent growing HepG2 cells were kept either untreated or were irradiated (500 μM H2O2, 24 h). Immediately after oxidative stress, cells were lysed and soluble and insoluble vimentin were assayed by Western blotting using anti-vimentin. Equal amounts (20 μg) of protein were loaded. c HepG2 cells were immunoprecipitated with anti-Hsp90 antibodies. The resulting pellets were subjected to SDS-PAGE and analyzed by immunoblotting with anti-vimentin antibodies. The signal intensity obtained from HepG2 cells without added GA was arbitrarily set to 1.0 (control)