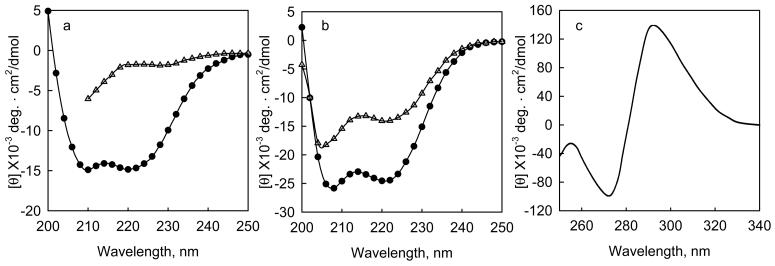
Figure 1. Changes in intrinsic and extrinsic circular dichroism bands upon the interaction of proteins with denaturants and ligands.
a, The change in mean residue ellipticity when Gn-HCl is added to a fragment of tropomodulin 1, Tmod1160-3591, 0.24 mg/ml (10 μM) in 100 mM NaCl, 10 mM sodium phosphate, pH 6.5 at 10 °C. (o) native protein, (Δ) protein + 3.6 M Gn-HCl. b. The change in mean residue ellipticity when calcium binds to the calcium binding subunit of cardiac troponin, TnC, 0.2 mg/ml, at 2 °C in 50 mM NaC1, 2 mM HEPES, pH 7.0, 0.5 mM DTT, with (Δ) 2mM EDTA, or (•) 3 mM CaCl22. c, Induced molar ellipticity when saturating sodium folate binds to dihydrofolate reductase from e coli.3. The enzyme is 18 μM in 100 mM NaCl, pH 7.2 at 27 °C.