Abstract
Aim
To explore immunological properties of human umbilical cord blood-derived stromal cells (hUCBDSC) and their effect on xenogeneic immune cells in vitro.
Methods
Immunological phenotype of freshly isolated and cryopreserved hUCBDSCs was evaluated by flow cytometry. Xenogeneic splenic T-cells were stimulated by phytohemaglutinin A (PHA) or dendritic cells in the absence or presence of hUCBDSCs. T-cell proliferation was measured by cell counting kit-8 after 7-day incubation. The proportion of apoptotic cells and CD4+CD25+Foxp3+ regulatory T-cells (Tregs) was determined in T-cells activated by PHA in the absence or presence of hUCBDSCs by flow cytometry. Phenotype of dendritic cells, cultured alone or with hUCBDSCs, was analyzed by flow cytometry.
Results
Levels of immune molecule expression on freshly isolated hUCBDSCs were as follows: human leukocyte antigen-I (HLA-I) (84.1 ± 2.9%), HLA-II (1.6 ± 0.3%), CD80 (0.8 ± 0.1%), CD86 (0.8 ± 0.1%), CD40 (0.6 ± 0.1%), and CD40L (0.5 ± 0.1%), which was not influenced by cryopreservation. T-cell proliferation in the presence of hUCBDSCs was significantly lower than that of positive control. The coculture led to a 10-fold increase (from 1.2 ± 0.3% to 12.1 ± 1.4%, P < 0.001) in the proportion of CD4+CD25+Foxp3+ regulatory T-cells (Tregs) and a reversion of mature dendritic cells, as indicated by the down-regulation of major histocompatibility complex (MHC)-II molecule (49.3% vs 25.9%, P = 0.001), CD80 (47.2% vs 23.3%, P = 0.001), and CD86 (40.6% vs 25.1%, P = 0.002). When subjected to annexin V binding and propidium iodide uptake assay, the hUCBDSCs did not show the ability to induce apoptosis of xenogeneic T-cells.
Conclusion
These results demonstrate low immunogenicity and immunomodulation effect of the hUCBDSCs. Reversion of mature dendritic cells and increase in Treg proportion, but not cell apoptosis, can possibly contribute to the suppression of xenogeneic T-cell proliferation by the hUCBDSCs.
Adherent spindle-shaped, fibroblastoid cells were isolated from the bone marrow by Friedenstein (1) and others (2-4), and are also referred to as multipotent mesenchymal stromal cells (MSC) (5) because of their self-renewal and multilineage differentiation capacity at the population level. One of the main biological functions of bone marrow MSCs is to regulate proliferation, differentiation, and maturation of hematopoietic cells through cell-to-cell interactions and secretion of cytokines and growth factors. Besides, MSCs also probably contribute to regulation of maturation, proliferation, and function of lymphocytes and have been shown to participate in the positive selection of T lymphocytes in the thymus in murine models (6-10).
In fact, a variety of studies with human, baboon, and murine MSCs have demonstrated the inability of MSCs to elicit a proliferative response of allogeneic lymphocytes, but instead they showed the capability of MSCs to suppress T lymphocyte activation and proliferation in vitro when stimulated by alloantigens, mitogens, and CD3 and CD28 antibodies (11-17). Different mechanisms are proposed to explain the suppressive effect of MSCs depending on the stimuli. Some animal models, related to alloreactive immunity, autoimmunity, or tumor immunity, have been established to examine the immunomodulatory function of MSCs in vivo. Graft vs host disease (GVHD), induced by donor T-cells, constitutes the most frequent complication after allogeneic hematopoietic cell transplantation (HCT) (18,19). Early and repeated systemic administration of ex-vivo expanded MSCs reduced the incidence and severity of GVHD in mice (20). It was shown clinically that the infusion of MSCs was safe and effective in the treatment of GVHD (21,22). MSCs have been an attractive candidate for cell therapy for GVHD.
Although MSCs have also been isolated from other tissues such as adipose tissue (23), placenta (24), amniotic fluid (25), and umbilical cord blood (26,27), most studies on immunomodulatory function focused on bone marrow MSCs. However, the bone marrow collection is an invasive procedure and the number and expansion capacity of bone marrow MSCs decline with age (28,29). Due to easy collection and younger age of donor cells, umbilical cord blood is one of the most attractive alternative sources of MSCs. Our laboratory has previously isolated a novel population of adherent fibroblast-like cells from human umbilical cord blood (hUCB) CD34+cells, called hUCB-derived stromal cells (hUCBDSCs) and confirmed their supportive effect on hematopoiesis in vitro (30).
Previous studies have usually investigated human MSCs-allogeneic immune cell reaction in vitro (11,14,31-33). To test the feasibility of replacing human immune cells with xenogeneic counterparts in vitro is of significance in establishing an animal model for further in vivo study of immunological properties of human MSCs, such as their inhibitory effect on GVHD. In the present study, we focused on immunological properties of hUCBDSCs and their effect on xenogeneic immune cells and demonstrated that hUCBDSCs suppressed xenogeneic T-cell activation induced by mitogen PHA and dendritic cells, in addition to its low immunogenicity per se, such as the lack of human leukocyte antigen-II (HLA-II) and some costimulator expression.
Material and methods
Isolation and expansion of hUCB-derived stromal cells
Full-term hUCB samples were harvested with informed consent of the mother. This study was approved by the local ethics committee. Cell culture was carried out as described in our previously published study (30).
Purification of T-cells from mouse spleen
Single-cell suspension of C57BL/6(H-2b/b) mouse spleen was prepared by gentle grinding and filtering through nylon mesh (40-µm diameter pores). Following the lysis of red blood cells with Tris-NH4Cl solution, splenocytes were resuspended in separation buffer (phosphate buffered saline, 0.5% bovine serum albumin, and 2mM disodium ethylenediamine tetraacetate). T-cells were enriched (>95% purity) by means of pan-T-cell isolation kits (Miltenyi Biotec, Auburn, CA, USA).
Cell cryopreservation and thawing
hUCBDSCs in log phase growth were suspended in culture medium containing 10% v/v dimethylsulfoxide and transferred to 1-mL cryovials. The cryovials were placed at 4°C for 30 minutes, at -20°C for 30 minutes, and at -70°C overnight. Next morning, cells were stored in liquid nitrogen. For cell resuscitation, cells were rapidly thawed in a water bath at 37°C and transferred into a centrifugation tube containing 8-mL culture medium. After centrifugation and removal of supernatant, cells were resuspended in 3-mL culture medium and cultured continually.
Induction of dendritic cells from mouse bone marrow
Dendritic cells from mouse bone marrow were obtained according to methods reported by Lutz (34). Femurs and tibias of 6 to 12-week-old female C57BL/6 or C57BL/6 × BALB/c F1(H-2b/d) mice were removed aseptically. The bone marrow was flushed with incomplete RPMI1640 using a syringe with a 0.45-mm diameter needle and washed once. Bone marrow leukocytes were seeded at about 2 × 106 per 100-mm dish in 10mL complete RPMI1640 medium containing 20 ng/mL recombinant mouse granulocyte-macrophage colony-stimulating factors (rmGM-CSF; Peprotech/Tebu, Frankfurt, Germany). Ten-milliliter fresh medium was added at day 3. At day 6 and day 8, half of the medium was changed. In some experiments, lypopolisaccharide (5 µg/mL; Sigma, Poole, UK) was added for another 48 hours to stimulate the maturation of dendritic cells.
Proliferative assays and mixed lymphocyte reaction
Splenic T-cells (1 × 105) from C57BL/6 mouse were seeded in triplicates onto 96-well plates in 200 µL complete RPMI 1640 medium in the absence or presence of mitogen phytohemaglutinin A (PHA; 10 µg/mL). hUCBDSCs were added in decreasing concentrations (105, 104, 103). After 7-day’s culture, cell proliferation was determined by cell counting kit-8 (Dojindo Laboratories, Kumamoto, Japan,). Briefly, 20 µL CCK-8 solution was added to each well, which was then incubated for another 4 hours. Optical density (OD) values were tested at wavelength of 450 nm by a microplate reader.
In mixed lymphocyte reaction (MLR) experiment, splenic T-cells (1 × 105) from C57BL/6 mouse were incubated with mature dendritic cells (1 × 105) from C57BL/6 × BALB/c F1 mouse bone marrow in a final volume of 200 µL per well in 96-well plate. Coculture with dendritic cells from syngeneic mouse was used as control. Stimulator dendritic cells were irradiated with 30 Gy of 60Co gamma radiation. hUCBDSCs were added in decreasing concentrations (105, 104, 103). Cell proliferation was measured by CCK-8 after 7-day incubation.
Cell coculture
Splenic T-cells from C57BL/6 mouse (1 × 106 per well) were plated into 12-well plates with PHA (10 µg/mL) in the absence or presence of hUCBDSCs (1 × 106 per well). At least 3 different human samples were used. Five days later, nonadherent cells were collected for determination of apoptotic T-cells and CD4+CD25+Foxp3+ regulatory T-cells (Tregs) using flow cytometry.
Mature bone marrow-dendritic cells, induced as mentioned above, were cultured alone or with hUCBDSCs from at least 3 different human samples. A series of surface markers on bone marrow-dendritic cells, including CD11c, MHC class II CD80, and CD86 were analyzed by flow cytometry on day 5.
Flow cytometry
Cells were collected, washed, and resuspended in phosphate-buffered saline. Then, they were incubated at 4°C for 30 minutes with the following conjugated monoclonal antibodies: FITC anti-human HLA-I, FITC anti-human HLA-II, FITC anti-human CD80, PE anti-human CD86, FITC anti-human CD40, FITC anti-human CD154, FITC anti-mouse MHC class II, FITC anti-mouse CD3, FITC anti-mouse CD80, FITC anti-mouse CD86, and PE anti-mouse CD11c (anti-HLA antibodies from BD Pharmingen, San Diego, CA, USA, and all others from eBioscience, San Diego, CA, USA). Equal aliquots of cells were labeled with isotype monoclonal antibodies to determine nonspecific reaction. Finally, cells were washed and assayed in a flow cytometer.
For determining of Tregs, cells were incubated with PE anti mouse-CD4 and Alexa Fluor®488 anti mouse CD25 (eBioscience) at 4°C for 30 minutes. Then, cells were washed, fixed, permeabilized, and stained with APC anti-mouse Foxp3 using APC anti-mouse Foxp3 staining set (eBioscience).
An annexin V binding and propidium iodide uptake assay (R&D Systems) was employed for apoptosis analysis. Briefly, the cells were washed once with “binding buffer” and then incubated with FITC labeled annexin V for 15 minutes in the dark at room temperature. Cells were washed once, resuspended in 0.5 mL binding buffer, counterstained with propidium iodide, and analyzed by flow cytometry.
Statistical analysis
Comparisons of 2 means were determined by t-test and multiple comparisons of means were determined by Tukey honestly significant difference test and Tamhane T2 of post hoc comparisons. Data were shown as mean ± standard deviation. Statistical significance level was set at P-values lower than 0.05. SPSS, version 13.0 (SPSS Inc., Chicago, IL, USA), was used to process the data.
Results
Immunophenotype of hUCBDSCs
Immunogenicity-associated surface markers HLA-I and HLA-II, as well as immune reaction-related costimulators CD80, CD86, CD40, and CD154 (CD40L), were analyzed on the successfully isolated hUCBDSCs from 5 different full-term hUCB samples. hUCBDSCs on passages 3-4 showing homogeneous spindle-shaped morphology were readily subjected to flow cytometry analysis. The results showed that 84.1 ± 2.9% of stromal cells expressed HLA-I, but there was nearly no expression of HLA-II (1.6 ± 0.3%). The levels of costimulator expression were as follows: CD80 (0.8 ± 0.1%), CD86 (0.8 ± 0.1%), CD40 (0.6 ± 0.1%), and CD40L (0.5 ± 0.1%) (Figure 1).
Figure 1.
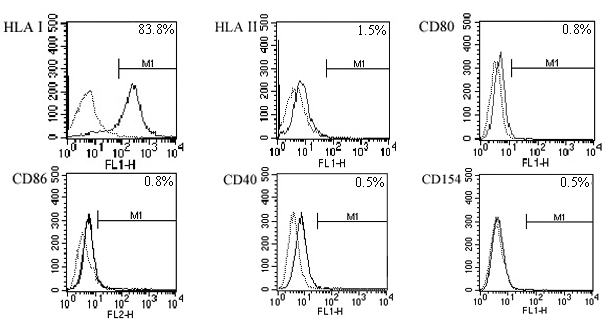
Expression of immunological cell surface markers on human umbilical cord blood-derived stromal cells (hUCBDSC). Most hUCBDSCs expressed human leukocyte antigen-l (HLA-I), whereas negative for HLA-Il antigens and costimulators such as CD80, CD86, CD40, or CD154. Solid curves represented staining with antigen specific antibodies, and dotted curves were used as isotype-matched negative control. The result showed was a representative of five independently tested samples.
Cell cryopreservation is a necessary procedure for assuring off-the-shelf availability of stromal cells. However, there remains the issue of biological properties of stromal cells after cryopreservation-thawing cycle (35). The hUCBDSCs that had been stored in liquid nitrogen were subjected to immunophenotype analysis and there was no significant difference in the expression of immune molecules between freshly isolated and cryopreserved hUCBDSCs (Table 1).
Table 1.
Comparison of immune molecule expression in freshly isolated and cryopreserved human umbilical cord blood-derived stromal cells (hUCBDSC)
| Immune molecules | Percentage of positive cells (%) | P* | |
|---|---|---|---|
| freshly isolated | with cryo-thaw cycle | ||
| HLA-I | 84.1 ± 2.9 | 87.3 ± 3.0 | 0.063 |
| HLA-II | 1.6 ± 0.3 | 1.7 ± 0.1 | 0.281 |
| CD80 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.108 |
| CD86 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.085 |
| CD40 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.182 |
| CD40L | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.291 |
*Student t-test.
hUCBDSCs suppressed xenogeneic T lymphocyte proliferation stimulated by PHA or dendritic cells
The purity of isolated splenic T-cells achieved 96.3 ± 3.5%, when using CD3 as an index. To test the ability of T-cell proliferation, hUCBDSCs were cocultured with splenic T lymphocytes from C57BL/6 mouse, while PHA-stimulated T lymphocytes were used as positive control. After 7-day coculture, OD-value indicated that T-cell proliferation in the presence of hUCBDSCs was significantly lower than that of PHA-stimulated T-cells (P = 0.001) and was comparable to that of control T-cells without stimulation. PHA-stimulated T-cell proliferation decreased correspondingly with the increased ratios of cocultured stromal cells to T-cells (Figure 2A).
Figure 2.
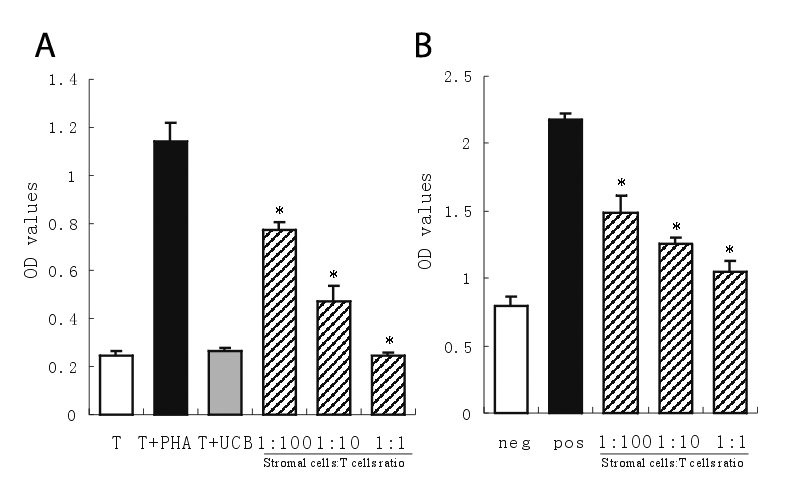
Effect of human umbilical cord blood-derived stromal cells (hUCBDSC) on xenogeneic T-cell response in vitro. (A) T-cells were cultured alone (T) or with hUCBDSCs (T+UBC). PHA-stimulated T-cell proliferation as showed in positive control (T+PHA). PHA-induced T-cell proliferation was inhibited proportionally to the fraction of added hUCBDSCs (hatched bars). (B) Allogeneic dendritic cells-induced T-cell proliferation was also suppressed by hUCBDSCs. Allogeneic dendritic cells-stimulated T-cell without hUCBDSCs served as positive control (pos) and syngeneic dendritic cells-stimulated T-cell served as negative control (neg). Allogeneic dendritic cell-stimulated T-cell proliferation was inhibited proportionally to the fraction of added hUCBDSCs (hatched bars). Data are shown as mean±SD of triplicates of 3 separate experiments using at least 3 different human samples. Asterisk indicates P < 0.01 vs PHA or allogeneic dendritic cells-stimulated T-cell.
In MLR experiment, allogeneic mature dendritic cells from C57BL/6 × BALB/c F1 mouse strongly stimulated proliferation of splenic T-cells from C57BL/6 mouse, compared with syngeneic mature dendritic cells. When hUCBDSCs were added at 0-day at different concentrations, T-cell proliferation induced by allogeneic mature dendritic cells was inhibited in a stromal cell concentration-dependent manner (Figure 2B).
All of the above mentioned results suggest that the addition of hUCBDSCs in in vitro culture did not evoke xenogeneic T-cell proliferation; on the contrary, it suppressed the proliferation induced by PHA or allogeneic dendritic cells.
Proportion of Tregs and apoptosis in T-cells cocultured with hUCBDSCs
To determine whether the suppressive effect of hUCBDSCs was associated with the induction of T-lymphocyte apoptosis, the percentage of apoptotic T lymphocytes was evaluated by flow cytometry after T lymphocyte coculture with stromal cells for 5 days. The result showed there was no significant difference in the proportion of apoptotic cells between T lymphocytes alone and cocultured T-cells (1.7 ± 0.2% vs 1.5 ± 0.1%, P = 0.100).
The proportion of Tregs plays an important role in down-regulating T lymphocyte response to stimulus. To test if the suppressive effect of hUCBDSCs on T lymphocytes was achieved through changing the proportions of T-cell subsets, T-cells that had cocultured with hUCBDSCs for 5 days were analyzed. There was a significant increase in the proportion of Tregs (12.1 ± 1.4% vs 1.2 ± 0.3%, P < 0.001) when T-cells were stimulated by PHA in the presence of hUCBDSCs compared with control (Figure 3).
Figure 3.
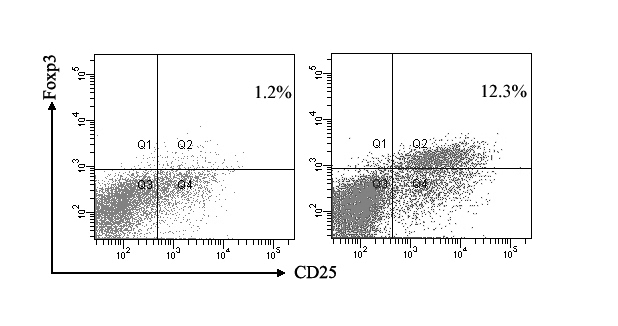
Percentage of Tregs in T-cells cultured alone or with human umbilical cord blood-derived stromal cells (hUCBDSC). The proportion of Tregs in T-cells was evaluated by flow cytometry from 3 experiments using at least 3 different human samples. Flow profile from one experiment was shown. It was indicated that there was a significant increase in the proportion of Treg in the presence of hUCBDSCs (12.3% vs 1.2%). T-cells alone (left); cocultured T-cells (right).
Effect of hUCBDSCs on mature bone marrow-dendritic cells
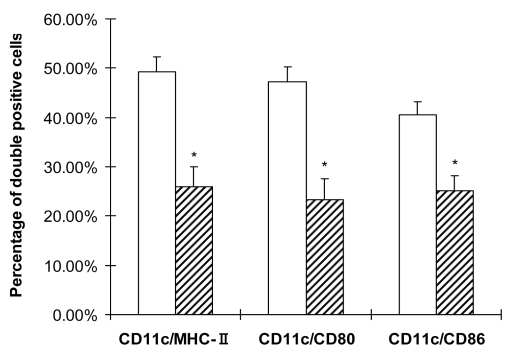
Mature dendritic cells are the most potent stimulus to T-cell response. In order to examine whether hUCBDSCs indirectly suppressed allogeneic dendritic cell-induced T-cell proliferation via dendritic cells, the immunological phenotype changes of mature bone marrow-dendritic cells were determined by flow cytometry after coculture with hUCBDSCs for 5 days. The results showed that the MHC-II molecule expression on mature bone marrow-dendritic cells was significantly down-regulated (49.3 ± 2.9% vs 25.9 ± 4.1%, P = 0.001). Expression of other costimulatory molecules, including CD80 (47.2 ± 3.1% vs 23.3 ± 4.3%, P = 0.001), and CD86 (40.6 ± 2.6% vs 25.1 ± 3.1%, P = 0.002), was also suppressed (Figure 4).
Figure 4.
Immunophenotype of mature bone marrow-dendritic cells cocultured with human umbilical cord blood-derived stromal cells (hUCBDSC). CD11c is known as a marker for murine dendritic cells. Mature bone marrow-dendritic cells, cultured alone or with hUCBDSCs, were stained simultaneously against CD11c (PE) and respectively in combination with MHC class II, CD80, or CD86 (FITC). The percentage of double positive cells was detected by flow cytometry. Results represented one of 3 independent experiments using at least 3 different human samples. Mature dendritic cells alone (blank bars); mature dendritic cells cocultured with hUCBDSCs (hatched bars). Asterisk indicates P < 0.01 vs mature dendritic cells alone.
Discussion
We demonstrated that the hUCBDSCs constitutively expressed HLA-I but not HLA-II and other costimulators, which was not influenced by cryopreservation, a common procedure in cell manipulation in clinic. In addition, hUCBDSCs did not elicit xenogeneic T-cell proliferation; instead, they suppressed the T-cell reaction in response to PHA or dendirtic cells. Generation of Tregs and reversion of mature dendritic cells, rather than induction of apoptosis of T-cells, might account for the possible mechanisms for the suppression of xenogeneic T-cell proliferation by hUCBDSCs.
HCT serves as the most effective treatment for high risk hematological malignancies (36). HLA-matched, related, or unrelated donors are the most suitable donors for HCT. But not all patients have available a suitable HLA-matched related or unrelated donor, and sometimes such donor cells cannot be provided timely for patients with aggressive malignancy. Recently, much attention has been focused on haploidentical stem cell transplantation, in which more than 90% patients share with their family donor 1 HLA haplotype for HLA-A, B, C, and DR but not the other. GVHD, a major cause of mortality and morbidity after HCT, is closely related to the degree of HLA incompatibility (36,37). Hence, GVHD remains a major obstacle for application of haploidentical stem cell transplantation. Our laboratory has been exerting efforts to reduce GVHD syndrome in clinical application of haploidentical stem cell transplantation. We isolated hUCBDSCs from hUCB CD34+cells and confirmed their similar characteristics with MSCs in morphology and surface markers (30). Preliminary studies have shown that co-transplantation of hUCBDSCs could promote engraftment of donor hematopoietic cell in mouse haploidentical stem cell transplantation model (not published). The benefit from co-transplanted hUCBDSCs suggests that hUCBDSCs may not be immunologically rejected, instead they probably help hematopoietic cell settle down in a way that is still unclear. By paying more attention to hUCBDSCs-xenogeneic immune cells reaction in vitro, this study obtained evidence for the application of the hUCBDSCs in mouse GVHD model associated with haploidentical stem cell transplantation and for treatment potential against GVHD as observed in MSCs.
In contrast to bone marrow, hUCB has many advantages: an easy and safe procurement, high frequency of immature stem and progenitor populations, the rapid availability of a cryopreserved off-the-shelf product, which is processed and quality controlled, and a reduced risk of immunological reactions (38). hUCB has been clinically considered as an alternative source of hematopoietic stem cells (HSC) for transplantation. Another population of non-hematopoietic cells in hUCB, constituting the microenviroment of HSCs, becomes more attractive in basic and transplantation research. Simmons et al (39) revealed that a subpopulation of bone marrow CD34+ cells comprising multipotent CFU-F activity (39,40) and depletion of CD34+ cells did not enhance the frequency of MSCs in umbilical cord blood (41). Our laboratory reported successful isolation of spindle-shaped fibroblastoid cells from CD34+ cells combining Dexter culture, called hUCBDSCs, and demonstrated its hematopoiesis-supporting property in vitro (30). It needs to be further investigated whether these differently named cells are the same population or have a common progenitor with HSCs.
We demonstrated that hUCBDSCs expressed high levels of HLA-I and negligible levels of HLA-II and other costimulators, which was similar to MSCs isolated from other sources. This immunological property was maintained after cryopreservation, which would make hUCBDSCs of substantial clinical interest. hUCBDSCs were not able to induce proliferation of xenogeneic T-cells from mouse in vitro. Accordingly, human MSCs did not induce xenoreactivity in vitro in previously unexposed immunocompetent SD rats, although these cells had been rejected when transplanted in rats (42). But in another study, human MSCs showed long-term engraftment after intrauterine transplantation in immunocompetent sheep (43). This suggests that hUCBDSCs present tolerance to xenogeneic T-cell response at least in vitro, probably due to lack of HLA-II expression and insufficient costimulator signals, as described for human MSCs.
In MLR experiment, T lymphocytes (responders) and dendritic cells (stimulators) were isolated and induced from C57BL/6(H-2b/b) mouse and C57BL/6 × BALB/c F1(H-2b/d) mouse, respectively, which share only 1 haplotype, to mimic GVHD occurring in haploidentical stem cell transplantation. In contrast to other in vitro studies, non-irradiated hUCBDSCs were added into MLR, considering that irradiation probably alters the biological activity of hUCBDSCs and their interaction with T-cells. The results showed that hUCBDSCs possessed the ability of inhibiting, in a cell dose-dependent manner, xenogeneic T-cell proliferation in response to PHA or dendritic cells, even with a small ratio (1 hUCBDSCs:100 lymphocytes). This result is in disagreement with Blank’s report (12), which showed that inhibitory effect of MSCs could not be observed with ratio of 1 MSCs:100 lymphocytes. Our xenogeneic model may account for the difference better than their allogeneic model.
T-cell unresponsiveness in the presence of hUCBDSCs is mainly attributed to peripheral clonal deletion (44) and suppression/regulation (45) mechanisms. Many studies found that MSCs had no effect on apoptosis of T-cells (12,46,47), expect 1 study which showed apoptosis of T-cells induced by MSCs due to the conversion of tryptophan to kynurenine (48). In agreement with the majority of studies, we found that the proportion of apoptotic T-cells in the presence of hUCBDSCs was comparative to that of control, indicating that peripheral clonal deletion mechanism could not account for the observed xenogeneic T-cell inability. CD4+Tregs, constitutively expressing CD25 and Foxp3, have been isolated from the thymus and periphery of mice, rats, and humans (49-51). These cells suppress activation and proliferation of T-cells in vitro and prevent development of autoimmune diseases when transplanted in animal experiments (52-58). Their main function in vivo is probably to regulate homeostatic proliferation of the peripheral T-cell pool (59). It has been demonstrated that the population of CD4+CD25+ regulatory cells was increased in PBMCs stimulated by mitogen in the presence of MSCs (31,33), however, one study showed that MSCs still can inhibit the proliferation of T-cells after depletion of CD4+CD25+ regulatory cells (17). In these experiments, regulatory cells were evaluated as CD4/CD25 double positive cells, which is not accurate considering that the transcription factor Foxp3 is the most specific marker of Tregs (60-62). So, we evaluated Tregs in T-cells as CD4+CD25+Foxp3+cells. When cocultured with hUCBDSCs, the percentage of Tregs in T-cells stimulated by PHA was significantly enhanced. The results suggest that hUCBDSCs inhibited xenogeneic T-cell proliferation in vitro probably through induction of Tregs differentiation, as observed in allogeneic setting, although the function of Tregs has not yet been demonstrated.
Dendritic cells, the most potent antigen-presenting cells with capacity to stimulate naive and memory T-cells, play a key role in the initiation of primary immune responses and in tolerance, depending on the activation and maturation stage. Mature dendritic cells induce immunogenic T-cell responses, whereas immature or semi-mature dendritic cells result in tolerance (63-65). MSCs prevented differentiation of monocyte and CD34+ cells into CD1a+ dendritic cells, and dendritic cells generated in the presence of MSCs showed no up-regulation of HLA-DR and other costimulatory molecules, resulting in impaired function to induce activation of T-cells (66-68). We demonstrated that hUCBDSCs also had the capacity to reverse xenogeneic mature dendritic cells to immature dendritic cells, of which MHC II and costimulatory molecules, including CD80 and CD86, are down-regulated. This may be another reason for the immunosuppressive effect of hUCBDSCs on xenogeneic T-cells.
In conclusion, hUCBDSCs, in addition to its low immunogenicity per se, suppressed xenogeneic T-cell activation induced by mitogen PHA and dendritic cells, probably due to increased Tregs proportion and reversed mature dendritic cells. The effect of hUCBDSCs on xenogeneic immune cells was similar to that of human MSCs on allogeneic immune cells. It is possible to further study immunomodulatory effects of hUCBDSCs on the prevention of GVHD in animal model, serving as a substitute for human GVHD associated with haploidentical stem cell transplantation.
Acknowledgments
This work was funded by grants from the National Natural Science Foundation (No. 30070327, No. 30500220, and No.30670890), natural science foundation project of CQ CSTC (No. 2009BA5011), and key discipline of medical science of Chongqing and special foundation for the “1520 project” of Xinqiao Hospital of Third Military Medical University.
References
- 1.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- 2.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 3.Owen M. Marrow stromal stem cells. J Cell Sci Suppl. 1988;10:63–76. doi: 10.1242/jcs.1988.supplement_10.5. [DOI] [PubMed] [Google Scholar]
- 4.Sale GE, Storb R. Bilateral diffuse pulmonary ectopic ossification after marrow allograft in a dog. Evidence for allotransplantation of hemopoietic and mesenchymal stem cells. Exp Hematol. 1983;11:961–6. [PubMed] [Google Scholar]
- 5.Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–5. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 6.Kurosaka D, LeBien TW, Pribyl JA. Comparative studies of different stromal cell microenvironments in support of human B-cell development. Exp Hematol. 1999;27:1271–81. doi: 10.1016/S0301-472X(99)00067-3. [DOI] [PubMed] [Google Scholar]
- 7.Roux E, Dumont-Girard F, Starobinski M, Siegrist CA, Helg C, Chapuis B, et al. Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity. Blood. 2000;96:2299–303. [PubMed] [Google Scholar]
- 8.Dittel BN, LeBien TW. Reduced expression of vascular cell adhesion molecule-1 on bone marrow stromal cells isolated from marrow transplant recipients correlates with a reduced capacity to support human B lymphopoiesis in vitro. Blood. 1995;86:2833–41. [PubMed] [Google Scholar]
- 9.Olsen NJ, Gu X, Kovacs WJ. Bone marrow stromal cells mediate androgenic suppression of B lymphocyte development. J Clin Invest. 2001;108:1697–704. doi: 10.1172/JCI13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Hisha H, Inaba M, Lian Z, Yu C, Kawamura M, et al. Evidence for migration of donor bone marrow stromal cells into recipient thymus after bone marrow transplantation plus bone grafts: A role of stromal cells in positive selection. Exp Hematol. 2000;28:950–60. doi: 10.1016/S0301-472X(00)00483-5. [DOI] [PubMed] [Google Scholar]
- 11.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.V99.10.3838. [DOI] [PubMed] [Google Scholar]
- 12.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 13.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–34. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 14.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 15.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–8. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- 16.Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–44. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 17.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 18.Mielcarek M, Martin PJ, Leisenring W, Flowers ME, Maloney DG, Sandmaier BM, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–62. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 19.Korngold R, Sprent J. Surface markers of T cells causing lethal graft-vs-host disease to class I vs class II H-2 differences. J Immunol. 1985;135:3004–10. [PubMed] [Google Scholar]
- 20.Yanez R, Lamana ML, Garcia-Castro J, Colmenero I, Ramirez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–91. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 21.Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389–98. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 23.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 24.In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–45. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 25.In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–9. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 26.Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625–34. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 27.Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–35. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 1999;17:171–7. doi: 10.1007/s007740050081. [DOI] [PubMed] [Google Scholar]
- 29.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–26. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Gao L, Chen X, Zhang X, Liu Y, Kong P, Peng X, et al. Human umbilical cord blood-derived stromal cell, a new resource of feeder layer to expand human umbilical cord blood CD34+ cells in vitro. Blood Cells Mol Dis. 2006;36:322–8. doi: 10.1016/j.bcmd.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 31.Maccario R, Podestr M, Moretta A, Cometa A, Comoli P, Montagna D, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–25. [PubMed] [Google Scholar]
- 32.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–13. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 34.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/S0022-1759(98)00204-X. [DOI] [PubMed] [Google Scholar]
- 35.Bieback K, Kluter H. Mesenchymal stromal cells from umbilical cord blood. Curr Stem Cell Res Ther. 2007;2:310–23. doi: 10.2174/157488807782793763. [DOI] [PubMed] [Google Scholar]
- 36.Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–9. doi: 10.1038/35077251. [DOI] [PubMed] [Google Scholar]
- 37.Sasazuki T, Juji T, Morishima Y, Kinukawa N, Kashiwabara H, Inoko H, et al. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program. N Engl J Med. 1998;339:1177–85. doi: 10.1056/NEJM199810223391701. [DOI] [PubMed] [Google Scholar]
- 38.Brunstein CG, Wagner JE. Umbilical cord blood transplantation and banking. Annu Rev Med. 2006;57:403–17. doi: 10.1146/annurev.med.57.051804.123642. [DOI] [PubMed] [Google Scholar]
- 39.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 40.Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84:4164–73. [PubMed] [Google Scholar]
- 41.Hutson EL, Boyer S, Genever PG. Rapid isolation, expansion, and differentiation of osteoprogenitors from full-term umbilical cord blood. Tissue Eng. 2005;11:1407–20. doi: 10.1089/ten.2005.11.1407. [DOI] [PubMed] [Google Scholar]
- 42.Grinnemo KH, Mlnsson A, Dellgren G, Klingberg D, Wardell E, Drvota V, et al. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg. 2004;127:1293–300. doi: 10.1016/j.jtcvs.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 43.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–6. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 44.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–56. doi: 10.1016/0092-8674(90)90420-J. [DOI] [PubMed] [Google Scholar]
- 45.Waldmann H, Cobbold S. Regulating the immune response to transplants. a role for CD4+ regulatory cells? Immunity. 2001;14:399–406. doi: 10.1016/S1074-7613(01)00120-0. [DOI] [PubMed] [Google Scholar]
- 46.Le Blanc K, Rasmusson I, Gotherstrom C, Seidel C, Sundberg B, Sundin M, et al. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004;60:307–15. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- 47.Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res. 2006;312:2169–79. doi: 10.1016/j.yexcr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 48.Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19:1597–604. doi: 10.1038/sj.leu.2403871. [DOI] [PubMed] [Google Scholar]
- 49.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–26. [PubMed] [Google Scholar]
- 50.Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25- subpopulations. J Immunol. 2000;165:3105–10. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 51.Stephens LA, Mottet C, Mason D, Powrie F. Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31:1247–54. doi: 10.1002/1521-4141(200104)31:4<1247::AID-IMMU1247>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 52.Sakaguchi S. Animal models of autoimmunity and their relevance to human diseases. Curr Opin Immunol. 2000;12:684–90. doi: 10.1016/S0952-7915(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 53.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/S1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 54.Singh B, Read S, Asseman C, Malmstrom V, Mottet C, Stephens LA, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065X.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 55.Shevach EM, McHugh RS, Piccirillo CA, Thornton AM. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev. 2001;182:58–67. doi: 10.1034/j.1600-065X.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- 56.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–40. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 58.Ermann J, Szanya V, Ford GS, Paragas V, Fathman CG, Lejon K. CD4(+)CD25(+) T cells facilitate the induction of T cell anergy. J Immunol. 2001;167:4271–5. doi: 10.4049/jimmunol.167.8.4271. [DOI] [PubMed] [Google Scholar]
- 59.Annacker O, Burlen-Defranoux O, Pimenta-Araujo R, Cumano A, Bandeira A. Regulatory CD4 T cells control the size of the peripheral activated/memory CD4 T cell compartment. J Immunol. 2000;164:3573–80. doi: 10.4049/jimmunol.164.7.3573. [DOI] [PubMed] [Google Scholar]
- 60.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–44. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 61.Picca CC, Caton AJ. The role of self-peptides in the development of CD4+ CD25+ regulatory T cells. Curr Opin Immunol. 2005;17:131–6. doi: 10.1016/j.coi.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 63.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 64.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 65.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/S1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 66.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–6. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 67.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–7. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 68.Zhang W, Ge W, Li C, You S, Liao L, Han Q, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–71. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]