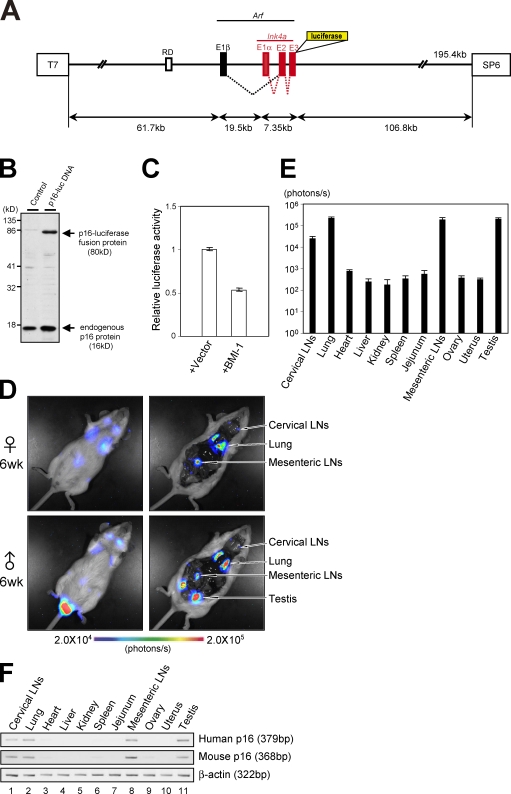
Figure 1.
Generation of human p16Ink4a reporter mice. (A) A large genomic DNA segment (195.4 kb) of human chromosome that contains the entire INK4a/ARF gene locus and surrounding sequences, including a putative DNA replication origin (RD) known to regulate p16Ink4a gene expression (Gonzalez et al., 2006), was engineered to express luciferase-tagged p16Ink4a. (B) BAC vector containing p16-luc DNA or empty BAC vector was transfected into 293T cells. Expression of the p16Ink4a-luciferase fusion protein was analyzed by Western blotting after selection with antibiotics. (C) BAC vector containing p16-luc DNA was introduced into 293T cells with or without BMI-1 expression plasmid along with 0.2 mg of MMLV (Moloney murine leukaemia virus)-lacZ plasmid. Luciferase activities were normalized by lacZ activities. Error bars indicate SD. (D) The 6-wk-old p16-luc mice were subjected to noninvasive BLI. Representative images of five different experiments are shown (left). The same mice were incised through the mouth and anus under anesthesia. Representative BLI data of five different experiments are shown. The color bar indicates photons with minimum and maximum threshold values. (E) Bioluminescence intensity emitted from the organs was graphed (log10 scale). The mean ± SD of five independent experiments is shown. (F) The levels of exogenous (human) p16Ink4a gene expression and endogenous (mouse) p16Ink4a gene expression in p16-luc mice were analyzed by semiquantitative RT-PCR. β-Actin was used as a loading control. Representative data of five different experiments are shown.