Figure 1.
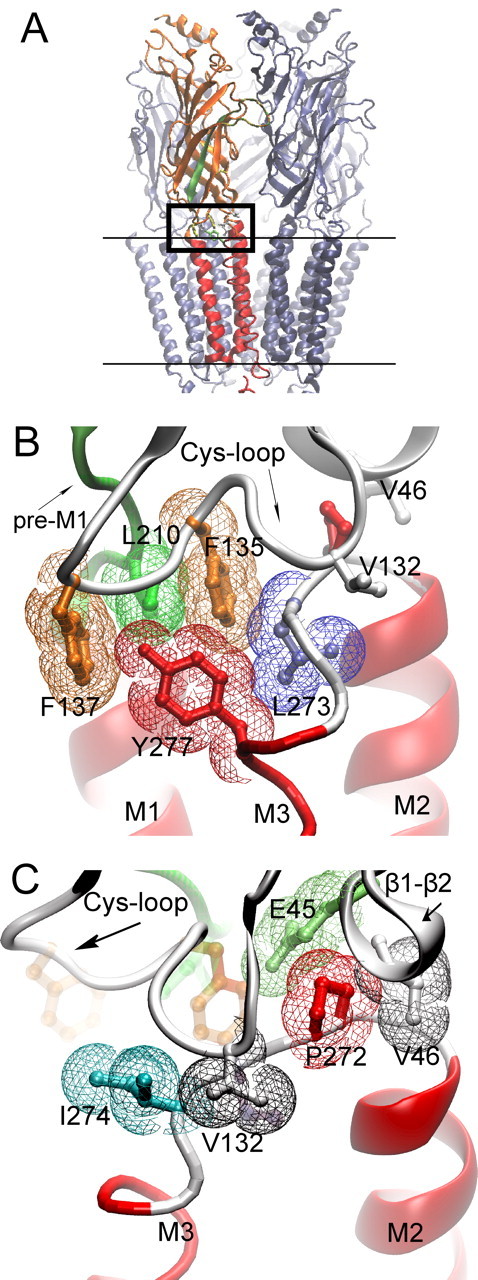
Cryo-electron microscopic structure of the Torpedo AChR (PDB code 2bg9). A, The pentameric AChR in the membrane (parallel lines) is shown with one of the two α-subunits highlighted; β-strands are orange and α-helices red. β-Strand 10 preceding transmembrane domain M1 region is green. The boxed region is the junction of extracellular and pore domains, shown at higher magnification in B and C. B, Residues from three converging regions of the α-subunit (Cys-loop, pre-M1 strand, M2–M3 linker) are shown in stick representation overlaid with colored van der Waals surfaces. C, The region in B is rotated to the left to illustrate residues with aliphatic side chains from the Cys-loop, β1–β2 loop, and M2–M3 linker.
