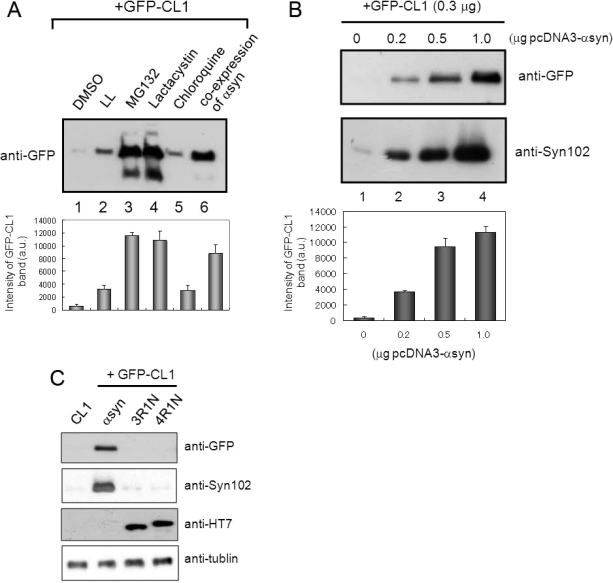
Figure 2.
Expression of α-synuclein inhibits proteasome activity in SH-SY5Y cells. (A) SH-SY5Y cells transfected with GFP-CL1 were treated with 1 μM LL, MG132, lactacystin, and chloroquine overnight. In the case of coexpression, cells were transfected with both GFP-CL1 and α-synuclein using FuGENE6. The cells were harvested, and the Triton X-100-insoluble and SDS-soluble fractions were prepared and analyzed by immunoblotting using an anti-GFP antibody. The results of quantitative analysis of the GFP-CL1 bands, expressed as means + SD (n = 3), are shown below the blot. (B) SH-SY5Y cells were transfected with GFP-CL1 (0.3 μg), α-synuclein (0, 0.2, 0.5, or 1 μg), and/or pcDNA3 empty vector (total plasmids = 1.3 μg) and incubated for 72 h. The cells were harvested, and then Tris-soluble (lower panels) and SDS-soluble fractions (upper panels) were prepared. Immunoblot analyses were performed using anti-GFP (upper panels) or anti-Syn102 (lower panels) antibodies. The results of quantitative analysis of the GFP-CL1 bands, expressed as means + SD (n = 3), are shown below the blot. (C) SH-SY5Y cells were transfected with both GFP-CL1 (0.3 μg) and α-synuclein (1 μg) or pcDNA3-tau 3R1N or 4R1N vector (1 μg) and incubated for 72 h. The cells were harvested, and the TX-insoluble fraction was analyzed by immunoblotting with anti-GFP antibody. The TS-soluble fraction was also analyzed by using anti-Syn102, anti-HT7, and anti-tubulin antibodies (as a loading control).