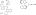Table 1.
Nucleophile-catalyzed cycloaddition of a ketene with a nitrosoarene: Effect of reaction parameters.
 | ||||
|---|---|---|---|---|
| Entry | Change from the "standard" conditions | Yield of 3 (%)[a] | ee of 3 (%)[b] | Yield of 4 (%)[c] |
| 1 | none | 90 | 79 | 3 |
| 2 | no (−)-1 | 9 | - | 30 |
| 3 | 5% (−)-2, instead of (−)-1 | 16 | −22 | 33 |
| 4 | 5% (+)-5, instead of (−)-1 | 19 | −35 | 61 |
| 5 | 5% (+)-6, instead of (−)-1 | 33 | <2 | 17 |
| 6 | toluene, instead of CH2Cl2 | 89 | 66 | 2 |
| 7 | THF, instead of CH2Cl2 | 71 | 78 | 1 |
| 8 | −20 °C | 81 | 73 | 6 |
| 9 | r.t. | 89 | 76 | 4 |
Determined by 1H NMR spectroscopy versus an internal standard.
A negative ee value signifies that the opposite enantiomer of 3 is formed preferentially.
Yield of isolated product (average of two experiments).