Table 2.
Nucleophile-catalyzed asymmetric cycloaddition of ketenes with a nitrosoarene.
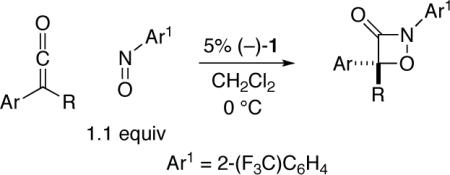 | ||||
|---|---|---|---|---|
| Entry | Ar | R | Yield (%)[a] | ee (%) |
| 1 | Ph | Me | 72 | 13 |
| 2 | 4-(MeO)C6H4 | Me | 60 | 3 |
| 3 | o-tolyl | Me | 90 | 90 |
| 4 | 2-(MeO)C6H4 | Me | 92 | 97 |
| 5 | 2-BrC6H4 | Me | 93 | 94 |
| 6 | Ph | Et | 88 | 80 |
| 7 | m-tolyl | Et | 86 | 78 |
| 8 | 1-naphthyl | Et | 86 | 78 |
| 9 | o-tolyl | Et | 90 | 96 |
| 10 | 2-(MeO)C6H4 | Et | 93 (88[b]) | >98 (>98[b]) |
| 11 | Ph | CH2CH2i-Pr | 86 | 79 |
| 12 | Ph | i-Bu | 90 | 91 |
| 13 | Ph | i-Pr | 85 | 92 |
| 14 | 4-ClC6H4 | i-Pr | 84 | 92 |
| 15 | 4-(MeO)C6H4 | i-Pr | 81 | 90 |
| 16 | 3-thienyl | i-Pr | 78 | 84 |
| 17 | Ph | cyclopentyl | 81 | 91 |
| 18 | Ph | cyclohexyl | 84 | 93 |
All data are the average of two experiments.
Yield of purified product.
Catalyst loading: 1%.
